Difference between revisions of "Chrysocolla"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 10: | Line 10: | ||
cedar green; copper silicate; gold solder; chryzokola (Pol.); crisocola (Port.); Chrysokoll (Deut.); chrysocolle (Fr.); chryssokolla (Gr.); crisocolla (It.); crisocola (Esp., Port.); chrysocolla (Ned.) | cedar green; copper silicate; gold solder; chryzokola (Pol.); crisocola (Port.); Chrysokoll (Deut.); chrysocolle (Fr.); chryssokolla (Gr.); crisocolla (It.); crisocola (Esp., Port.); chrysocolla (Ned.) | ||
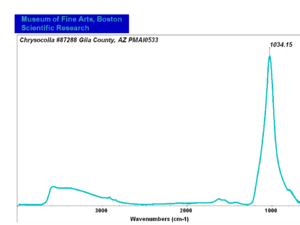
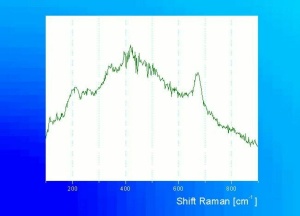
| − | [[[SliderGallery rightalign|chrysocolla531.jpg~Raman]]] | + | [[[SliderGallery rightalign|Chrysocolla, Gila County AZ.PNG~FTIR (MFA)|chrysocolla531.jpg~Raman]]] |
== Other Properties == | == Other Properties == | ||
Revision as of 09:00, 8 October 2019
Description
A sky blue mineral composed of hydrated copper silicate. Chrysocolla is a secondary copper ore that has also been used as a Gemstone and a blue-green pigment. It has been gathered or mined as a semiprecious stone since 3000 BCE. Chrysocolla is mined in England (Cornwall, Cumberland), Congo, Zaire, Chile, and the U.S. (Pennsylvania, Arizona, New Mexico and Utah). The translucent to opaque stone is sky-blue in its natural state, but appears green when ground into a fine powder. Chrysocolla has been found as a pigment in wall painting at Kizil in Turkistan and in Twelfth Dynasty Egyptian tombs (Gettens and Stout 1966). In the 16th and 17th centuries, it was used as a watercolor pigment called cedar green. Chrysocolla is stable to light but is decomposed by acids, alkalis, and heat.
Synonyms and Related Terms
cedar green; copper silicate; gold solder; chryzokola (Pol.); crisocola (Port.); Chrysokoll (Deut.); chrysocolle (Fr.); chryssokolla (Gr.); crisocolla (It.); crisocola (Esp., Port.); chrysocolla (Ned.)
Other Properties
Luster = vitreous to dull. Fracture = uneven to conchoidal. Streak = white or pale blue-green. Pigment particles are anisotropic with high birefringence, straight extinction and are pleochroic changing from pale green to colorless
| Composition | CuSiO3-nH2O |
|---|---|
| Mohs Hardness | 2 - 4 |
| Density | 2.0-2.8 |
| Refractive Index | 1.575; 1.598; 1.597 |
Hazards and Safety
No significant hazards
Additional Information
° R. J. Gettens and G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966.
° Mineralogy Database: Chrysocolla
Comparisons
Characteristics of Common Blue Pigments
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density =2.4, ref. index=1.575; 1.598; 1.59
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Kurt Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975 Comment: p. 52
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref.index.1.575; 1.598; 1.597
- Encyclopedia Britannica, http://www.britannica.com Comment: Chrysocolla. Retrieved May 25, 2003, from Encyclopædia Britannica Premium Service. (tech info-ref. index = 1.45; 1.55)
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Chrysocolla (Accessed Sept. 2, 2005) hardness=2.5-3.5
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 232
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000