Difference between revisions of "Microcrystalline wax"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 10: | Line 10: | ||
Examples include: Multiwax W445; Bareco® 85C (185F); Be Square® 77-92C (170-197F); Bareco® Victory 74C (165F); Cosmolloid 85C (185F); Renaissance Wax | Examples include: Multiwax W445; Bareco® 85C (185F); Be Square® 77-92C (170-197F); Bareco® Victory 74C (165F); Cosmolloid 85C (185F); Renaissance Wax | ||
| + | == Applications == | ||
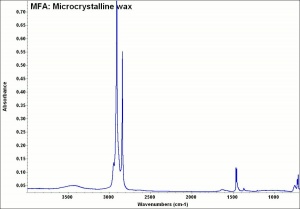
| + | [[[SliderGallery rightalign|MFA- Microcrystalline wax.jpg~FTIR]]] | ||
| − | + | == Risks == | |
| − | == | + | == Physical and Chemical Properties == |
Iodine value=0, acid value=0, saponification value=0 | Iodine value=0, acid value=0, saponification value=0 | ||
| Line 32: | Line 34: | ||
[[media:download_file_20.pdf|Properties of Natural Waxes]] | [[media:download_file_20.pdf|Properties of Natural Waxes]] | ||
| − | |||
Revision as of 20:25, 19 May 2020
Description
A high molecular weight hydrocarbon wax that has a fine crystalline structure. Microcrystalline waxes were first made in the late 1930s by Baker Petrolite in Barnsdall, Oklahoma. It is the remaining fraction of Paraffin wax after the lower molecular weight waxes are removed. Microcrystalline wax is chemically inert, and in general, a stronger adhesive than paraffin wax. It can be softened by adding Mineral oil or Petroleum solvent. Microcrystalline wax does not emulsify easily but can be modified with a catalyst to produce an oxidized, emulsifiable form that is used in hard, self-polishing floor wax. Microcrystalline wax is used in laminating paper and foils as well as for polishes. It polishes to a glass clear, smooth, non-sticky finish.
See also Mineral wax.
Synonyms and Related Terms
petrolatum wax; cera microcristalina (Esp.); cire microcristalline (Fr.); cera microcristallina (It);
Examples include: Multiwax W445; Bareco® 85C (185F); Be Square® 77-92C (170-197F); Bareco® Victory 74C (165F); Cosmolloid 85C (185F); Renaissance Wax
Applications
Risks
Physical and Chemical Properties
Iodine value=0, acid value=0, saponification value=0
| Melting Point | 60-93 |
|---|---|
| Density | 0.915-0.941 |
| Refractive Index | 1.441 |
Comparisons
Sources Checked for Data in Record
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 591
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- A History of Technology, Charles Singer, E.J. Holmyard, A.R. Hall (eds.), Clarendon Press, Oxford, Volume 1: From Early times to Fall of Ancient Empires, 1954
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: melting point=71-89C, density=0.928-0.941, ref. index = 1.441, iodine value=0, acid value=0, saponification value=0
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Microcrystalline_wax (Accessed Feb. 10, 2006)
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000