Difference between revisions of "Acetic anhydride"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless liquid with a strong [[ | + | A colorless liquid with a strong [[acetic acid]] odor. Acetic anhydride is a strong acetylating agent and is used primarily for the production of [[cellulose%20acetate|cellulose acetate]] fibers and plastics. It is also used in the production of pharmaceuticals, dyes, perfumes, and explosives. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
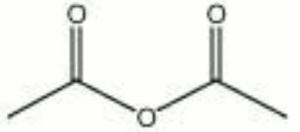
[[[SliderGallery rightalign|acetic anhydride.jpg~Chemical structure]]] | [[[SliderGallery rightalign|acetic anhydride.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Readily combustible, fire hazard. Overexposure may result in edema, eye irritation, cough, skin burns and dermatitis. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=S25119A&productDescription=ACETIC+ANHYDRIDE+500ML&vendorId=VN00115888&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Reacts with water slowly to form acetic acid. Reacts with alcohols to form the corresponding acetate. Soluble in chloroform and ether. | Reacts with water slowly to form acetic acid. Reacts with alcohols to form the corresponding acetate. Soluble in chloroform and ether. | ||
| Line 37: | Line 43: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Revision as of 11:17, 19 October 2020
Description
A colorless liquid with a strong Acetic acid odor. Acetic anhydride is a strong acetylating agent and is used primarily for the production of Cellulose acetate fibers and plastics. It is also used in the production of pharmaceuticals, dyes, perfumes, and explosives.
Synonyms and Related Terms
acetic acid anhydride; acetyl oxide; acetic oxide; ethanoic anhydride
Risks
Readily combustible, fire hazard. Overexposure may result in edema, eye irritation, cough, skin burns and dermatitis.
ThermoFisher: SDS
Physical and Chemical Properties
Reacts with water slowly to form acetic acid. Reacts with alcohols to form the corresponding acetate. Soluble in chloroform and ether.
| Composition | (CH3CO)2O |
|---|---|
| CAS | 108-24-7 |
| Melting Point | -73 |
| Density | 1.080 |
| Molecular Weight | mol. wt. = 102.2 |
| Refractive Index | 1.3904 |
| Boiling Point | 139 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: ref. index=1.3904
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.8
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.389