Difference between revisions of "Anhydrite"
| Line 12: | Line 12: | ||
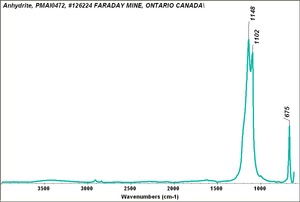
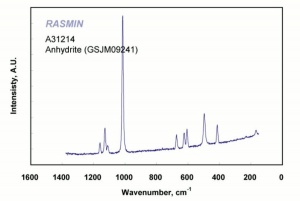
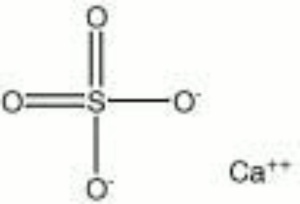
[[[SliderGallery rightalign|Anhydrite PMA.TIF~FTIR (PMA)|anhydriteRS.jpg~Raman|anhydrite.jpg~Chemical structure]]] | [[[SliderGallery rightalign|Anhydrite PMA.TIF~FTIR (PMA)|anhydriteRS.jpg~Raman|anhydrite.jpg~Chemical structure]]] | ||
| − | == | + | == Physical and Chemical Properties == |
Orthorhombic crystals but usually massive. Perfect cleavage in three directions. Sometimes fluorescent, especially after heating. | Orthorhombic crystals but usually massive. Perfect cleavage in three directions. Sometimes fluorescent, especially after heating. | ||
Revision as of 10:24, 24 April 2022
Description
A naturally occurring mineral of anhydrous calcium sulfate that is often found in gypsum deposits. Anhydrite was first identified as a mineral in deposits at Innsbruck, Austria in 1794. Other major deposits occur in Canada (Ontario), and the U.S. (Arizona, New Mexico). Anhydrite can be transparent or translucent and has a lustrous sheen. It occurs in several colors such as white, gray, blue, pink, red, and lavender. Anhydrite was used in ancient Egypt for carved objects and vessels, many of which are in the shape of animals (Fay, 1998). Powdered anhydrite has also been found as a filler in the gesso grosso ground layers of Italian paintings and polychromed sculpture. Currently, the colorless, inert pigment is often used as a filler in paper, paints, and plastics.
Synonyms and Related Terms
anhydrous calcium sulfate; Anhydrit (Deut.); anhydrite (Fr.); anhidrita (Esp.); anydritis (Gr.); anidrite (It.); anhydriet (Ned.); anhydryt (Pol.); anidrite (Port.); blue marble; anhydrite white;
Physical and Chemical Properties
Orthorhombic crystals but usually massive. Perfect cleavage in three directions. Sometimes fluorescent, especially after heating.
Fracture = uneven. Luster = vitreous to pearly. Streak = white to gray.
Under cross polarsparticles usually show bright second order interference
| Composition | CaSO4 |
|---|---|
| CAS | 7778-18-9 |
| Mohs Hardness | 3.0 - 3.5 |
| Melting Point | 1450 |
| Density | 2.9-3.0 |
| Molecular Weight | mol. wt. = 136.14 |
| Refractive Index | 1.570; 1.575; 1.614 |
Hazards and Safety
Can alter to gypsum in humid conditions.
Mallinckrodt Baker: MSDS
Additional Information
° B.Fay ""Egyptian Duck Flasks of Blue Anhydrite" in Metropolitan Museum Journal, 33:23-48, 1998.
° Mineralogy Database: Anhydrite
Additional Images
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.569-1.574; beta=1.574-1.579; gamma=1.609-1.618
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.93; refractive index = 1.570; 1.614; 1.575
- Encyclopedia Britannica, http://www.britannica.com Comment: "anhydrite" [Accessed December 4, 2001].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 38
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Anhydrite
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: transparent white pigment