Difference between revisions of "Cobalt yellow"
| Line 1: | Line 1: | ||
[[File:aureolin C100x.jpg|thumb|Aureolin]] | [[File:aureolin C100x.jpg|thumb|Aureolin]] | ||
== Description == | == Description == | ||
| − | + | [[File:Cobaltyellow C100x.jpg|thumb|Cobalt yellow (with filler)\\ | |
A brilliant, transparent yellow pigment commonly known by the name aureolin. Cobalt yellow is composed of potassium cobaltinitrite. It was discovered by N.W. Fischer in Germany in 1848. Cobalt yellow was introduced as an artists' pigment in 1852 (Saint-Evre) and began selling commercially in the U.S. in 1861. Aureolin is a permanent pigment, but may discolor to brown when mixed with indigo in the presence of hydrogen sulfide. Because of its poor hiding power, cobalt yellow is most often used in watercolor and tempera paints, for oil-based glazes and as a colorant in glass. | A brilliant, transparent yellow pigment commonly known by the name aureolin. Cobalt yellow is composed of potassium cobaltinitrite. It was discovered by N.W. Fischer in Germany in 1848. Cobalt yellow was introduced as an artists' pigment in 1852 (Saint-Evre) and began selling commercially in the U.S. in 1861. Aureolin is a permanent pigment, but may discolor to brown when mixed with indigo in the presence of hydrogen sulfide. Because of its poor hiding power, cobalt yellow is most often used in watercolor and tempera paints, for oil-based glazes and as a colorant in glass. | ||
| Line 9: | Line 9: | ||
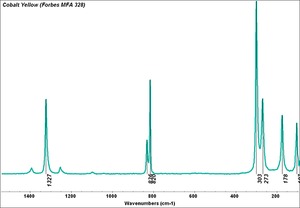
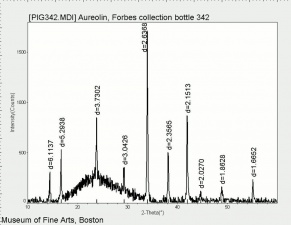
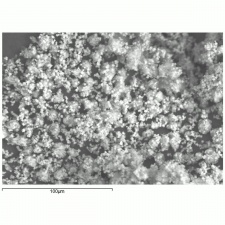
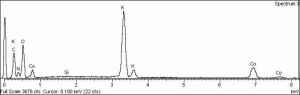
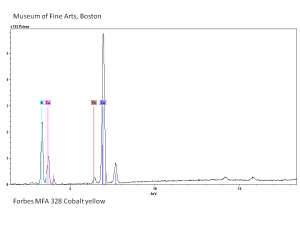
[[[SliderGallery rightalign|Cobalt Yellow (Forbes MFA 328) resize.tif~Raman (MFA)|PIG342.jpg~XRD|f342sem.jpg~SEM|f342edsbw.jpg~EDS|Slide37 FC328.PNG~XRF]]] | [[[SliderGallery rightalign|Cobalt Yellow (Forbes MFA 328) resize.tif~Raman (MFA)|PIG342.jpg~XRD|f342sem.jpg~SEM|f342edsbw.jpg~EDS|Slide37 FC328.PNG~XRF]]] | ||
| + | |||
| + | == Risks == | ||
| + | |||
| + | * Ingestion may cause poisoning with decreased blood pressure, headaches, nausea, vomiting and cyanosis. | ||
== Other Properties == | == Other Properties == | ||
| − | Slightly soluble in water. Insoluble in ethanol, ether and carbon disulfide. Soluble in acids producing a pink solution. Decomposes in alkalis evolving nitrogen oxide fumes. | + | * Slightly soluble in water. |
| − | + | * Insoluble in ethanol, ether and carbon disulfide. | |
| − | Fine, dendritic crystals. Yellow in transmitted light. Isotropic in polarized light. | + | * Soluble in acids producing a pink solution. |
| + | * Decomposes in alkalis evolving nitrogen oxide fumes. | ||
| + | * Fine, dendritic crystals. | ||
| + | * Yellow in transmitted light. | ||
| + | * Isotropic in polarized light. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 25: | Line 33: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.8 | + | | 1.8 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 42: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M.Cornman, "Cobalt Yellow", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press; Cambridge, 1986. | |
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: introduced as a paint pigment by Saint-Evre in Paris 1852 | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: introduced as a paint pigment by Saint-Evre in Paris 1852 | ||
| Line 60: | Line 54: | ||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 Comment: appeared commercially in the 1860s | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 Comment: appeared commercially in the 1860s | ||
| − | * | + | * Pigments through the Ages: http://webexhibits.org/pigments/indiv/overview/coyellow.html - gives chemical name as Cobalt(II) aluminum nitrite - states unchanged in acids but will turn black in sodium sulfide and brown in caustic soda |
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
Revision as of 13:14, 30 May 2022
Description
[[File:Cobaltyellow C100x.jpg|thumb|Cobalt yellow (with filler)\\ A brilliant, transparent yellow pigment commonly known by the name aureolin. Cobalt yellow is composed of potassium cobaltinitrite. It was discovered by N.W. Fischer in Germany in 1848. Cobalt yellow was introduced as an artists' pigment in 1852 (Saint-Evre) and began selling commercially in the U.S. in 1861. Aureolin is a permanent pigment, but may discolor to brown when mixed with indigo in the presence of hydrogen sulfide. Because of its poor hiding power, cobalt yellow is most often used in watercolor and tempera paints, for oil-based glazes and as a colorant in glass.
Synonyms and Related Terms
potassium cobaltinitrite; Pigment Yellow 40; CI 77357; aureolin; Kobaltgelb (Deut.); jaune de cobalt (Fr.); auréoline (Fr.); giallo di cobalto (It.); amarillo de cobalto (Esp.); aureolina (Esp.); Aureolin (Deut.); Primulin (Deut.); kitrino toy kobaltioy (Gr.); giallo di cobalto (aureolina) (It.); aureoline (Ned.); amarelo de cobalto (Port.); potassium hexanitrocobaltate; cobalt potassium nitrite; cobaltic potassium nitrite; Fischer's yellow;
Risks
- Ingestion may cause poisoning with decreased blood pressure, headaches, nausea, vomiting and cyanosis.
Other Properties
- Slightly soluble in water.
- Insoluble in ethanol, ether and carbon disulfide.
- Soluble in acids producing a pink solution.
- Decomposes in alkalis evolving nitrogen oxide fumes.
- Fine, dendritic crystals.
- Yellow in transmitted light.
- Isotropic in polarized light.
| Composition | K3[Co(NO2)6].3H2O |
|---|---|
| CAS | 13782-01-9 |
| Density | 1.8 g/ml |
| Molecular Weight | mol. wt. = 452.26 |
| Refractive Index | 1.72 - 1.76 |
Resources and Citations
- M.Cornman, "Cobalt Yellow", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press; Cambridge, 1986.
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: introduced as a paint pigment by Saint-Evre in Paris 1852
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: Introduced as artists pigment in 1861 by Winsor & Newton; ref. index = 1.72-1.76
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 Comment: appeared commercially in the 1860s
- Pigments through the Ages: http://webexhibits.org/pigments/indiv/overview/coyellow.html - gives chemical name as Cobalt(II) aluminum nitrite - states unchanged in acids but will turn black in sodium sulfide and brown in caustic soda
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2493
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997