Difference between revisions of "Smithsonite"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 3: | Line 3: | ||
Hard, dense, often shiny, mineral composed of [[zinc%20carbonate|zinc carbonate]]. Smithsonite was the princiapl source for zinc prior to 1880. It has been found in Greece (Laurium), Germany (Aachen), Austria (Carinthia), Poland (Bytom, Tarnowskie Góry), Italy (Sardinia), Rhodesia (Broken Hill mine) and the U.S. (Pennsylvania, Arkansas, Utah, New Mexico, Colorado, California). It was named for James Smithson, founder of the Smithsonian Institution. Smithsonite can be white, gray, green, blue, yellow, purple, pink, or brown. It occurs as a secondary mineral in the oxidized zones of hydrothermal ore deposits. | Hard, dense, often shiny, mineral composed of [[zinc%20carbonate|zinc carbonate]]. Smithsonite was the princiapl source for zinc prior to 1880. It has been found in Greece (Laurium), Germany (Aachen), Austria (Carinthia), Poland (Bytom, Tarnowskie Góry), Italy (Sardinia), Rhodesia (Broken Hill mine) and the U.S. (Pennsylvania, Arkansas, Utah, New Mexico, Colorado, California). It was named for James Smithson, founder of the Smithsonian Institution. Smithsonite can be white, gray, green, blue, yellow, purple, pink, or brown. It occurs as a secondary mineral in the oxidized zones of hydrothermal ore deposits. | ||
| − | |||
[[File:ps20613smithsonite.jpg|thumb|Smithsonite]] | [[File:ps20613smithsonite.jpg|thumb|Smithsonite]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 11: | Line 10: | ||
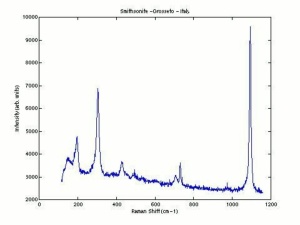
[[[SliderGallery rightalign|Smithsoniteitaly1.jpg~Raman]]] | [[[SliderGallery rightalign|Smithsoniteitaly1.jpg~Raman]]] | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | Luster = adamantine to pearly Streak = white Cleavage = perfect in three directions | + | * Luster = adamantine to pearly |
| + | * Streak = white | ||
| + | * Cleavage = perfect in three directions | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 21: | Line 22: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.3-4.5 | + | | 4.3-4.5 g/ml |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Smithsonite.shtml Smithsonite] | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "smithsonite" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "smithsonite" [Accessed December 3, 2002]. (color picture) |
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Smithsonite (Accessed Sept. 17, 2005) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 12:47, 31 May 2022
Description
Hard, dense, often shiny, mineral composed of Zinc carbonate. Smithsonite was the princiapl source for zinc prior to 1880. It has been found in Greece (Laurium), Germany (Aachen), Austria (Carinthia), Poland (Bytom, Tarnowskie Góry), Italy (Sardinia), Rhodesia (Broken Hill mine) and the U.S. (Pennsylvania, Arkansas, Utah, New Mexico, Colorado, California). It was named for James Smithson, founder of the Smithsonian Institution. Smithsonite can be white, gray, green, blue, yellow, purple, pink, or brown. It occurs as a secondary mineral in the oxidized zones of hydrothermal ore deposits.
Synonyms and Related Terms
calamine (former name); zinc spar; Zincspat (Deut.)
Physical and Chemical Properties
- Luster = adamantine to pearly
- Streak = white
- Cleavage = perfect in three directions
| Composition | ZnCO3 |
|---|---|
| Density | 4.3-4.5 g/ml |
Resources and Citations
- Mineralogy Database: Smithsonite
- Encyclopedia Britannica, http://www.britannica.com Comment: "smithsonite" [Accessed December 3, 2002]. (color picture)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Smithsonite (Accessed Sept. 17, 2005)