Difference between revisions of "Sodium bisulfite"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
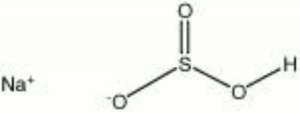
| − | + | [[[SliderGallery rightalign|sodium bisulfite.jpg~Chemical structure]]] | |
White crystals or powder. Sodium bisulfite is used as a [[disinfectant|disinfectant]] and [[bleaching%20agent|bleach]], especially for wool and leather. It is also used as a bleach, digestive aid, and [[antichlor%20agent|antichlor]] in papermaking vats. Sodium bisulfite is also used in photographic developing solutions and dye baths as a reducing agent. | White crystals or powder. Sodium bisulfite is used as a [[disinfectant|disinfectant]] and [[bleaching%20agent|bleach]], especially for wool and leather. It is also used as a bleach, digestive aid, and [[antichlor%20agent|antichlor]] in papermaking vats. Sodium bisulfite is also used in photographic developing solutions and dye baths as a reducing agent. | ||
| Line 7: | Line 7: | ||
sodium acid sulfite; sodium hydrogen sulfite; sodium metabisulfite; sodium pyrosulfite | sodium acid sulfite; sodium hydrogen sulfite; sodium metabisulfite; sodium pyrosulfite | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Noncombustible. | ||
| + | * Contact causes irritation and burns. | ||
| + | * Toxic by ingestion. | ||
| + | * Reacts with acids to evolve toxic sulfur dioxide fumes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC419440010&productDescription=SODIUM+BISULFITE%2C+REAGENT+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water. Insoluble in ethanol. | Soluble in water. Insoluble in ethanol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 150 | + | | 150 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.48 | + | | 1.48 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 40: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 785 | ||
Latest revision as of 13:50, 1 June 2022
Description
White crystals or powder. Sodium bisulfite is used as a Disinfectant and bleach, especially for wool and leather. It is also used as a bleach, digestive aid, and antichlor in papermaking vats. Sodium bisulfite is also used in photographic developing solutions and dye baths as a reducing agent.
Synonyms and Related Terms
sodium acid sulfite; sodium hydrogen sulfite; sodium metabisulfite; sodium pyrosulfite
Risks
- Noncombustible.
- Contact causes irritation and burns.
- Toxic by ingestion.
- Reacts with acids to evolve toxic sulfur dioxide fumes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Insoluble in ethanol.
| Composition | NaHSO3 |
|---|---|
| CAS | 7631-90-5 |
| Melting Point | 150 C |
| Density | 1.48 g/ml |
| Molecular Weight | mol. wt. = 104.06 |
| Refractive Index | 1.474, 1.526, 1.685 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8731
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index= 1.474, 1.526, 1.685