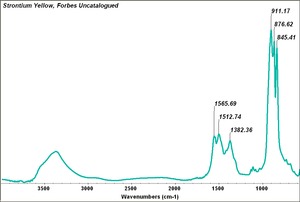
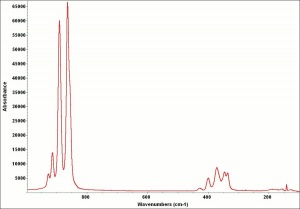
Difference between revisions of "Strontium yellow"
| Line 10: | Line 10: | ||
== Risks == | == Risks == | ||
| − | May turn green in strong sunlight. | + | * May turn green in strong sunlight. |
| − | + | * Toxic by ingestion or inhalation. | |
| − | Toxic by ingestion or inhalation. Skin contact may cause allergies. Carcinogenic. | + | * Skin contact may cause allergies. Carcinogenic. |
| − | + | * American Elements: [file:///C:/Users/mderr/Downloads/strontium-chromate-7789-06-2_sds%20(1).pdf SDS] | |
| − | American Elements: [file:///C:/Users/mderr/Downloads/strontium-chromate-7789-06-2_sds%20(1).pdf SDS] | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 28: | Line 27: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.89 | + | | 3.89 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
Latest revision as of 11:33, 6 June 2022
Description
A pale yellow pigment composed of Strontium chromate. Strontium yellow was used in oil paints in the late 19th and early 20th centuries. It has good opacity and stability to light and heat. But because of its high hiding power, strontium chromate was not used in watercolors. Commercially strontium chromate was often mixed with other pigments: a mixture with Prussian blue was sold as green cinnabar and a mixture with Barium yellow and Zinc yellow then sold under the name lemon yellow or citron yellow. Currently, strontium yellow is used in anticorrosive coating and pyrotechnics.
Synonyms and Related Terms
strontium chromate; Pigment Yellow 32; CI 77839; CAS #: 7789-06-2; Strontiumgelb (Deut.); jaune de strontium (Fr.); amarillo de estroncio (Esp.); giallo di stronzio (It.); strontiumgeel (Ned.); amarelo de estrôncio (Port.); lemon yellow; strontium chrome; strontaine yellow; strontian yellow; citron yellow;
Risks
- May turn green in strong sunlight.
- Toxic by ingestion or inhalation.
- Skin contact may cause allergies. Carcinogenic.
- American Elements: SDS
Physical and Chemical Properties
Soluble in dilute acids and hot water. Slightly soluble in cold water.
| Composition | SrCrO4 |
|---|---|
| CAS | 7789-06-2 |
| Density | 3.89 g/ml |
| Molecular Weight | mol. wt. = 203.62 |
| Refractive Index | 1.92; 2.01 |
Resources and Citations
- H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- M. Doerner, The Materials of the Artist, Harcourt, Brace & Co., 1934
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 610
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9001
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000