Difference between revisions of "Ronnel"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 3: | Line 3: | ||
A non-US trademark for a light brown, solid [[insecticide|insecticide]]. Ronnel is an organophosphate compound that works as a cholinesterase inhibitor. It has been used on livestock and dogs to kill fleas, ticks, and flies. Ronnel was formerly used in household formulations for [[cockroach|cockroaches]], [[termite|termites]], and ants. The crystalline powder was patented by Bayer in 1948. | A non-US trademark for a light brown, solid [[insecticide|insecticide]]. Ronnel is an organophosphate compound that works as a cholinesterase inhibitor. It has been used on livestock and dogs to kill fleas, ticks, and flies. Ronnel was formerly used in household formulations for [[cockroach|cockroaches]], [[termite|termites]], and ants. The crystalline powder was patented by Bayer in 1948. | ||
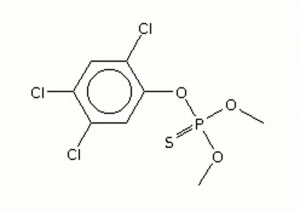
| − | + | [[[SliderGallery rightalign|ronnelir.jpg~FTIR|ronnelstr.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
phosphorothioic acid; O,O-dimethyl-O-(2,4,5 trichlorophenyl)phosphorothioate; fenchlorophos; fenchlorphos; dimethyl trichlorophenyl thiophosphate; trichlormetaphos | phosphorothioic acid; O,O-dimethyl-O-(2,4,5 trichlorophenyl)phosphorothioate; fenchlorophos; fenchlorphos; dimethyl trichlorophenyl thiophosphate; trichlormetaphos | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Skin contact causes irritation. | ||
| + | * ChemService: [http://cdn.chemservice.com/product/msdsnew/External/English/N-11952%20English%20SDS%20US.pdf SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in most organic solvents. Insoluble in water. | Soluble in most organic solvents. Insoluble in water. | ||
| Line 23: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 41 | + | | 41 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.485 | + | | 1.485 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 97 | + | | 97 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 51: | Line 49: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * | + | * Spectrum Laboratory - Chemical Fact sheet at http://www.speclab.com/compound/c299843.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:22, 27 June 2022
Description
A non-US trademark for a light brown, solid Insecticide. Ronnel is an organophosphate compound that works as a cholinesterase inhibitor. It has been used on livestock and dogs to kill fleas, ticks, and flies. Ronnel was formerly used in household formulations for cockroaches, termites, and ants. The crystalline powder was patented by Bayer in 1948.
Synonyms and Related Terms
phosphorothioic acid; O,O-dimethyl-O-(2,4,5 trichlorophenyl)phosphorothioate; fenchlorophos; fenchlorphos; dimethyl trichlorophenyl thiophosphate; trichlormetaphos
Risks
- Toxic by ingestion and inhalation.
- Skin contact causes irritation.
- ChemService: SDS
Physical and Chemical Properties
Soluble in most organic solvents. Insoluble in water.
| Composition | (CH3O)2P(S)OC6H2Cl3 |
|---|---|
| CAS | 299-84-3 |
| Melting Point | 41 C |
| Density | 1.485 g/ml |
| Molecular Weight | mol. wt. = 321.57 |
| Boiling Point | 97 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8415
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Spectrum Laboratory - Chemical Fact sheet at http://www.speclab.com/compound/c299843.htm