Difference between revisions of "Copal"
| Line 1: | Line 1: | ||
| − | [[File:02.222-E11509CR-d1.jpg|thumb|]] | + | [[File:02.222-E11509CR-d1.jpg|thumb|Devotion panel<br>MFA# 02.222]] |
== Description == | == Description == | ||
| Line 12: | Line 12: | ||
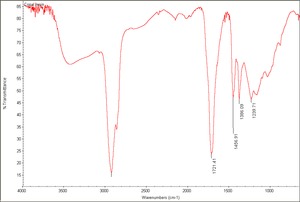
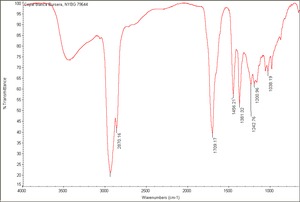
[[[SliderGallery rightalign|Copal resin.TIF~FTIR(MFA)|Copal blanca Bursera, NYBG 79644.TIF~FTIR(MFA) Blanca Bursera]]] | [[[SliderGallery rightalign|Copal resin.TIF~FTIR(MFA)|Copal blanca Bursera, NYBG 79644.TIF~FTIR(MFA) Blanca Bursera]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Contact and inhalation may cause irritation. | ||
| + | * Combustible, burning with a bright flame, dense smoke and strong smell. | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
After melting, copals are soluble in oil and turpentine. Soft copals are partially soluble in ethanol, chloroform, glacial acetic acid. Most copals fluoresce white in short-wave UV light. | After melting, copals are soluble in oil and turpentine. Soft copals are partially soluble in ethanol, chloroform, glacial acetic acid. Most copals fluoresce white in short-wave UV light. | ||
| Line 19: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 180-340 | + | | 180-340 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.04-1.13 | + | | 1.04-1.13 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| 1.528-1.545 | | 1.528-1.545 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
[[media:download_file_101.pdf|Properties of Natural Resins]] | [[media:download_file_101.pdf|Properties of Natural Resins]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
<gallery> | <gallery> | ||
| − | File:15-28_Copal_glass.jpg|Copal | + | File:15-28_Copal_glass.jpg|Copal glass |
</gallery> | </gallery> | ||
| − | + | ==Resources and Citations== | |
| − | == | + | * K.van den Berg, J.van der Horst, J.Boon, "Recognition of Copals in Aged Resin/oil Paints and Varnishes" in ICOM Preprints, Lyon, 1999. p.855-861. |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: melting points vary from 180-340C | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: melting points vary from 180-340C | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Copal." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Copal." Accessed 5 Jan. 2004. |
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| Line 81: | Line 74: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Copal |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.04-1.14; ref. index = 1.528 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.04-1.14; ref. index = 1.528 | ||
Revision as of 12:17, 4 July 2022
Description
A large variety of hard, natural resins obtained directly from trees such as Trachylobium species (Africa), Hymenaea courbaril (South America) and Agathis australis (New Zealand). Copals are also obtained as fossil resins from Zaire and Zanzibar. These fossil resins are very hard and almost completely insoluble. Copals are diterpenoid resins that contain communic acids, communol, resene, and volatile oil. They range in color from colorless to a bright yellow-brown. Classified as copal dur, the hardest copal resin is Zanzibar. Sierra Leone, Kauri, and Congo are of medium hardness (le demi-dur). Manila and Borneo are soft copals (le tendre). The oldest, most fossilized, resins are the hardest. Copal resins may be purchased as large lumps or small tears. They were used as oil varnishes for coaches in the 18th and 19th centuries but they darken and become insoluble with age. They also have been used in commercial varnishes, Linoleum, Oilcloth, and as an Amber substitute.
Synonyms and Related Terms
copal (Esp.); copale (It); Zanzibar; Demerara; Benguela; Sierra Leone; Mozambique; red Angola; white Angola; Congo; kauri; Manila; Pontianak; Madagascar; Accra; Loango; Gaboon; Borneo; Singapore; South American; Cochin; Brazilian; Bursera; Benin, swamp gum; anime; cowrie
Risks
- Contact and inhalation may cause irritation.
- Combustible, burning with a bright flame, dense smoke and strong smell.
Physical and Chemical Properties
After melting, copals are soluble in oil and turpentine. Soft copals are partially soluble in ethanol, chloroform, glacial acetic acid. Most copals fluoresce white in short-wave UV light.
| Melting Point | 180-340 C |
|---|---|
| Density | 1.04-1.13 g/ml |
| Refractive Index | 1.528-1.545 |
Comparisons
Additional Images
Resources and Citations
- K.van den Berg, J.van der Horst, J.Boon, "Recognition of Copals in Aged Resin/oil Paints and Varnishes" in ICOM Preprints, Lyon, 1999. p.855-861.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: melting points vary from 180-340C
- Encyclopedia Britannica, http://www.britannica.com Comment: "Copal." Accessed 5 Jan. 2004.
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.545 (Congo)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 227
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 2581
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Wikipedia: http://en.wikipedia.org/wiki/Copal
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.04-1.14; ref. index = 1.528
- Paintings Specialty Group, Painting Conservation Catalog, Wendy Samet (ed.), AIC, Washington, DC, 1998