Difference between revisions of "Pyrope"
Jump to navigation
Jump to search
| Line 8: | Line 8: | ||
garnet; Cape ruby; Bohemian garnet; rhodolite (violet); piropo (Esp., Port.); Pyrop (Deut.); pyroop (Ned.) | garnet; Cape ruby; Bohemian garnet; rhodolite (violet); piropo (Esp., Port.); Pyrop (Deut.); pyroop (Ned.) | ||
| + | ==Physical and Chemical Properties== | ||
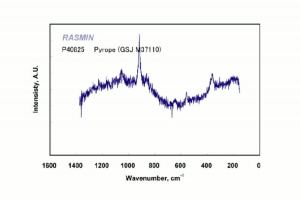
[[[SliderGallery rightalign|pyrope.jpg~Raman]]] | [[[SliderGallery rightalign|pyrope.jpg~Raman]]] | ||
| − | |||
| − | |||
| − | |||
* Fracture = conchoidal | * Fracture = conchoidal | ||
* Luster = vitreous to resinous | * Luster = vitreous to resinous | ||
Revision as of 12:37, 20 August 2022
Description
A transparent, ruby-red Garnet composed of magnesium aluminum silicate where the magnesium is partially replaced with calcium and iron. The color of pyrope ranges from a deep red to almost black. Gemstone quality pyropes are mined in the Czech Republic, South Africa, and Australia.
Synonyms and Related Terms
garnet; Cape ruby; Bohemian garnet; rhodolite (violet); piropo (Esp., Port.); Pyrop (Deut.); pyroop (Ned.)
Physical and Chemical Properties
- Fracture = conchoidal
- Luster = vitreous to resinous
- Streak = colorless to white
- Birefringence = isotropic
- Pleochroism = none
- UV fluorescence = inert.
- Composition = 3MgO-Al2O3-3SiO2
- Mohs Hardness = 7.0 - 7.5
- Density = 3.78 g/ml
- Refractive Index = 1.714-1.742
Comparisons
Properties of Common Gemstones
Resources and Citations
- Mineralogy Database: Pyrope
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 354
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Encyclopedia Britannica, http://www.britannica.com Comment: "pyrope." Accessed 14 Sept. 2001 .
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Pyrope (Accessed Sept. 14, 2005)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998