Difference between revisions of "Quenstedtite"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
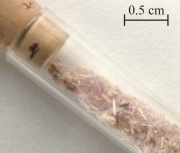
| − | Tabular to prismatic | + | Tabular to prismatic lavender color crystals of hydrated [[ferrous%20sulfate|ferrous sulfate]]. Quenstedtite mineral was named for Friedrich A. Von Quenstedt, a German paleontologist and stratigrapher. The attached infrared spectrum is for a reference sample (Harvard 109520) obtained from Chuquicamata, Chile. |
| − | == | + | ==Physical and Chemical Properties== |
| − | Triclinic crystal system. Cleavage is perfect in one direction | + | * Triclinic crystal system. |
| + | * Cleavage is perfect in one direction | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 17: | Line 18: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.147 | + | | 2.147 g/ml |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | * Website | + | * Website: http://labs.sci.qut.edu.au/minerals/mineral%20general%20descriptions/Q/quenstedtitepcd.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:56, 27 September 2022
Description
Tabular to prismatic lavender color crystals of hydrated Ferrous sulfate. Quenstedtite mineral was named for Friedrich A. Von Quenstedt, a German paleontologist and stratigrapher. The attached infrared spectrum is for a reference sample (Harvard 109520) obtained from Chuquicamata, Chile.
Physical and Chemical Properties
- Triclinic crystal system.
- Cleavage is perfect in one direction
| Composition | Fe2(SO4)3.10H2O |
|---|---|
| Mohs Hardness | 2.5 |
| Density | 2.147 g/ml |