Difference between revisions of "Malic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|malic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|malic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC125252500&productDescription=DL-MALIC+ACID%2C+99%2B%25+250GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in water, methanol, diethyl ether, acetone and ethanol. Slightly soluble in ether. Insoluble in benzene. | Soluble in water, methanol, diethyl ether, acetone and ethanol. Slightly soluble in ether. Insoluble in benzene. | ||
| Line 24: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 131-132 | + | | 131-132 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.601 | + | | 1.601 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 55 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 55 | ||
Latest revision as of 13:35, 1 October 2022
Description
White, water-soluble crystals that occur naturally in apples and other fruits. Malic acid is used as a chelating and buffering agent. The weak acid is also used to acidify food and to age wine.
Synonyms and Related Terms
apple acid; hydroxysuccinic acid; hydroxybutanedioic acid
Risks
- Combustible.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, methanol, diethyl ether, acetone and ethanol. Slightly soluble in ether. Insoluble in benzene.
pH = 2.2 (0.1 N solution)
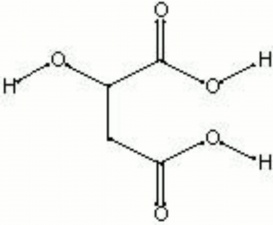
| Composition | COOHCH2CH(OH)COOH |
|---|---|
| CAS | 617-48-1 (dl form) |
| Melting Point | 131-132 C |
| Density | 1.601 g/ml |
| Molecular Weight | mol. wt. = 134.09 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 55
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5747
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 2.2 (0.1 N solution)