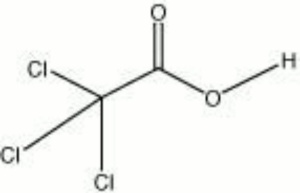
Trichloroacetic acid
Jump to navigation
Jump to search
Description
White, deliquescent crystals precipitate proteins and is used as a reagent for the detection of Albumin. Trichloroacetic acid is used in the manufacture of pharmaceuticals and herbicides.
Synonyms and Related Terms
TCA
Other Properties
Soluble in water, ethanol, ether. pH = 1.2 (for 0.1 M solution).
| Composition | CCl3COOH |
|---|---|
| CAS | 76-03-9 |
| Melting Point | 57-58 |
| Density | 1.6298 |
| Molecular Weight | mol. wt. = 163.39 |
| Boiling Point | 196-197 |
Hazards and Safety
Toxic by ingestion and inhalation. Highly corrosive on contact. Decomposes to form chloroform, hydrochloric acid, carbon dioxide, carbon monoxide.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9756