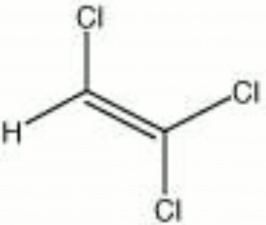
Trichloroethylene
Jump to navigation
Jump to search
Description
Colorless liquid with a chloroform odor. Trichloroethylene has been used as a solvent for fats, oils, waxes, resins, Rubber, plastics, paints, and varnishes. It was widely used for degreasing and dry-cleaning, but by 1990 its usage was banned in many states.
Synonyms and Related Terms
trichloroethene; 1,1,2-trichloroethylene; ethylene trichloride; westrosol; Tri-Clene [DuPont]; Trethylene; Chlorylene;
Risks
- Nonflammable but decomposes with heat to produce toxic fumes.
- Potential carcinogen.
- Toxic by inhalation.
- Usage prohibited in some states.
- Skin contact causes irritation.
- Millipore Sigma: MSDS
Physical and Chemical Properties
Miscible with organic solvents. Insoluble in water.
| Composition | CHCl:CCl2 |
|---|---|
| CAS | 79-01-6 |
| Melting Point | -73 C |
| Density | 1.456-1.462 g/ml |
| Molecular Weight | mol. wt.= 131.4 |
| Refractive Index | 1.4735 |
| Boiling Point | 86.7 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 303
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9769; ref. index=1.4735
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.475