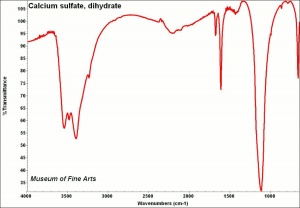
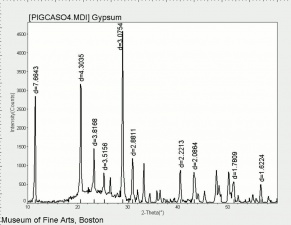
Calcium sulfate, dihydrate
Revision as of 13:31, 18 May 2022 by MDerrick (talk | contribs) (→Physical and Chemical Properties)
Description
White lumps or powder that is commonly called by its mineral name of Gypsum. Calcium sulfate dihydrate is used in the manufacture of portland cement. Gypsum is also used as a filler and pigment in paints, enamels, glazes and paper.
Synonyms and Related Terms
native calcium sulfate; precipitated calcium sulfate; gypsum; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; Pigment White 25
Risks
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water. Slightly soluble in glycerol. Insoluble in most organic solvents.
- Gypsum fluoresces purple.
| Composition | CaSO4-2H2O |
|---|---|
| CAS | 13397-24-5 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 100-150 C |
| Density | 2.32-2.36 g/ml |
| Molecular Weight | mol. wt. = 172.2 |
| Refractive Index | 1.520; 1.530; 1.523 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753