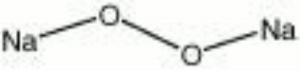
Sodium peroxide
Revision as of 12:03, 27 April 2013 by (username removed)
Description
Yellowish-white, hygroscopic powder. Sodium peroxide is used industrially as a agent bleach for paper, textiles, bones, feathers, ivory, wood, wax, sponges, and coral. It is also used as an disinfectant in germicidal soaps. Sodium peroxide is used for rocket propulsion and to produce oxygen aboard submarines.
Synonyms and Related Terms
sodium dioxide; sodium superoxide; Solozone
Other Properties
Reacts exothermically with water producing sodium hydroxide and oxygen.
| Composition | Na2O2 |
|---|---|
| CAS | 1313-60-6 |
| Melting Point | 460 |
| Density | 2.805 |
| Molecular Weight | mol. wt. = 77.98 |
| Boiling Point | 657 |
Hazards and Safety
Strong oxidizing agent. Reacts violently with water, alcohols, acids, powdered metals and organic materials. Skin contact causes irritation and burns.
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 566
- Richard S. Lewis, Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8800
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998