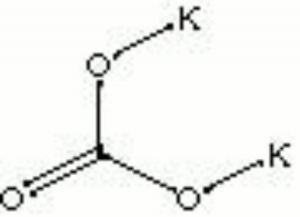
Potassium carbonate
Description
White deliquescent powder. Potassium carbonate is used in the manufacture of glass, ceramics, smalt, and soap. It is also used in printing inks, process engraving, and lithography and in tanning and finishing leather. In a closed environment, a saturated solution of potassium carbonate will form an equilibrium at a humidity relative humidity of about 44% (20C).
Synonyms and Related Terms
salts of tartar, pearl ash; potash; salt of wormwood; carbonate of potass; american ashes; Kaliumcarbonat (Deut.); Pottasche (Deut.); carbonato de potasio (Esp.); wglan potasu (Pol.)
Other Properties
Soluble in water. Insoluble in ethanol.
Deliquescent point at 20C is 44 % RH (see salt solutions saturated salt solutions)
| Composition | K2CO3 |
|---|---|
| CAS | 584-08-7 |
| Melting Point | 891 |
| Density | 2.428 |
| Molecular Weight | mol. wt. = 138.21 |
| Refractive Index | 1.426, 1.531, 1.541 |
Hazards and Safety
Noncombustible. Skin contact causes irritation. Ingestion may be harmful.
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 630
- Richard S. Lewis, Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7781
- Website address 1, Website address 1 Comment: Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Potassium_carbonate (Accessed Mar. 1, 2006)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.426, 1.531, 1.541