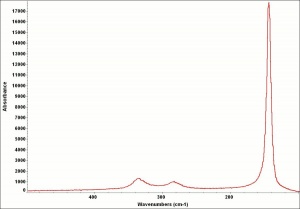
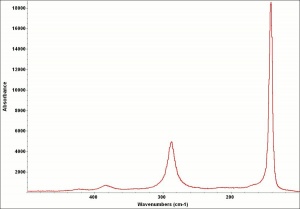
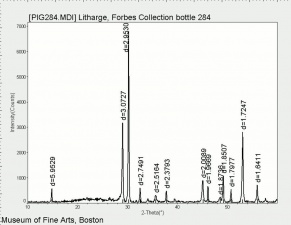
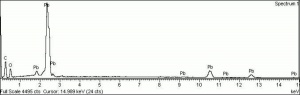
Lead monoxide
Description
A heavy yellow powder that is named litharge and massicot. Litharge is the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. The name massicot is used for both the native mineral as well as the lead monoxide product made by heating carbonate, basic lead carbonate to 300C. Lead monoxide has been used a a drier in oil and as a low-fire flux in making ceramics and glass. It is also used as a yellow pigment in paints and glazes. Massicot was used as an artists pigment from the 15th through the 18th centuries (Mayer 1969). Thin layers of lead monoxide are used to produce iridescent colors on brass and bronze. It has also been used as a filler for natural rubber and to produce artificial tortoiseshell and horn. Litharge is mixed with glycerol to make plumber's cement.
Synonyms and Related Terms
lead (II) oxide; monxido de plomo (Esp.); monoxyde de plomb (Fr.); litharge (Fr.); monxido de chumbo (Port.); litharge; massicot; yellow lead oxide; plumbous oxide; yellow lead oxide; protoxide of lead; semi-vitreous oxide of lead; lithargyron; plumbum ustum
Other Properties
Soluble in acetic acid, dilute nitric acid and alkalis. Insoluble in water and ethanol. Turns gray on exposure to sulfur fumes.
| Composition | PbO |
|---|---|
| CAS | 1317-36-8 |
| Melting Point | 888 |
| Density | 9.53 |
| Molecular Weight | mol. wt. = 223.2 |
Hazards and Safety
Toxic by inhalation or ingestion. Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen.
LINK: International Chemical Safety Card
Additional Information
R. Mayer, A Dictionary of Art Terms and Techniques, Harper and Row, New York, 1969.
Authority
- R. J. Gettens, G.L. Stout, R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 545
- Ralph Mayer, Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5433