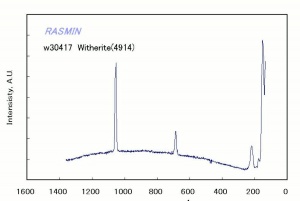
Barium carbonate
Description
A heavy white powder that occurs in nature as the mineral witherite. Barium carbonate is used as a pigment in the manufacture of paints, glazes and synthetic marble. When hydroxide barium hydroxide is used as an alkalizing agent for paper, it can react with dioxide carbon dioxide and precipitate as barium carbonate to provide an alkaline reserve. Both barium hydroxide and barium carbonate are highly toxic. Barium carbonate is used commercially in rat poison, bricks, cement, and mortar.
Synonyms and Related Terms
witherite; carbonate de baryum (Fr.); Pigment White 10; CI 77099
Other Properties
Soluble in acid. Insoluble in water.
Flat tablets that are colorless under plane-polarized light; high brefringence with complete extinction; interference colors are often seen. Fluoresces a light blue color in both long and short wave UV
| Composition | BaCO3 |
|---|---|
| CAS | 513-77-9 |
| Mohs Hardness | 3.5 |
| Melting Point | 811 |
| Density | 4.275 |
| Molecular Weight | mol. wt. = 197.34 |
| Refractive Index | 1.529; 1.677; 1.676 |
| Boiling Point | 1300 |
Hazards and Safety
Highly toxic by ingestion. Contact with skin and membranes may cause irritation.
Mallinckrodt Baker: MSDS
Comparisons
Characteristics of Common White Pigments
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density 4.3 and ref. index 1.529; 1.677; 1.676
- G.S.Brady, G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 84
- Richard S. Lewis, Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 966
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Barium_carbonate (Accessed Sept 2, 2005; hardness = 3.5, sp = 4.3)
- Book and Paper Group, Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 Comment: transparent white pigment