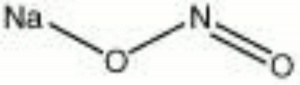Sodium nitrite
Revision as of 12:08, 27 April 2013 by (username removed)
Description
White to pale yellow hygroscopic crystals. Sodium nitrite is used in dyeing textiles with dye developed dyes. It is also used as a fixative for color photographs. In a closed environment, a saturated solution of sodium nitrite will form an equilibrium at a relative humidity of about 65% (20C).
Synonyms and Related Terms
nitrous acid sodium salt; erinitrit; nitrite
Other Properties
Soluble in water. Slightly soluble in ethanol.
Deliquescent point at 20C is 65.3 % RH (see salt solutions saturated salt solutions)
| Composition | NaNO2 |
|---|---|
| CAS | 7632-00-0 |
| Melting Point | 271 |
| Density | 2.157 |
| Molecular Weight | mol. wt. = 69.0 |
Hazards and Safety
Carcinogenic in test animals. Strong oxidizing agent. Fire risk in contact with oxidizing materials. Used as an antidote for cyanide poisoning.
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8793