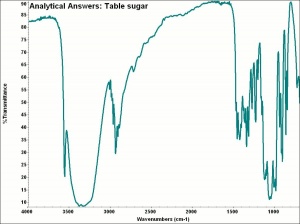
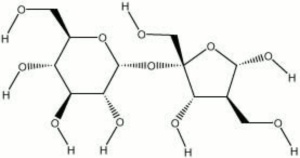
Sucrose
Description
Hard, white, crystals with a sweet taste. Sucrose occurs naturally in sugar cane, sugar beets, sugar maple, and sorghum. It is extracted with water and purified with lime or carbon. Sucrose is a disaccharide that hydrolyzes to glucose and fructose. It is primarily used as a sweetening agent in foods and drinks. It has also been used in the manufacture of polyurethane foams, printing inks, and transparent soaps. Sucrose has been used in conjunction with mannitol for the impregnation of waterlogged wood (Morgos and Imazu, 1993).
Synonyms and Related Terms
table sugar; saccharose; cane sugar; beet sugar
Other Properties
Soluble in water. Slightly soluble in ethanol, methanol, glycerol, pyridine.
Chars with the smell of caramel.
| Composition | C12H22O11 |
|---|---|
| CAS | 57-50-1 |
| Melting Point | 160 (dec) |
| Density | 1.5877 |
| Molecular Weight | mol. wt. = 342.3 |
Hazards and Safety
Combustible. High concentrations may cause irritation.
Mallinckrodt Baker: MSDS
Additional Information
A Morgos, S.Imazu "A Conservation Method for Waterlogged Wood using a Sucrose-Mannitol Mixture" ICOM Preprints, Washington DC, 1993, p.266-272.
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- John S. Mills, Raymond White, The Organic Chemistry of Museum Objects, Butterworth Heineman, London, 2nd ed., 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9051