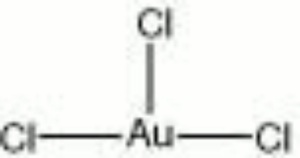
Gold trichloride
Jump to navigation
Jump to search
Description
Dark orange crystals that decompose with light or heat. An aqueous solution is called chlorauric acid or acid gold trichloride. Gold trichloride is used as a toner for black and white photographs. It is also used as a glaze for ceramics, enameling glass and making Ruby glass. Other uses of gold trichloride include gold plating and the production of fine gold powder.
Synonyms and Related Terms
auric chloride; auric trichloride; gold (III) chloride
Other Properties
Soluble in water, ethanol and ether.
| Composition | AuCl3 |
|---|---|
| CAS | 13453-07-1 |
| Density | 3.9 |
| Molecular Weight | mol. wt. = 303.32 |
| Boiling Point | 229 |
Hazards and Safety
Decomposes with heat. Very hygroscopic. Contact, inhalation, and ingestion cause irritation and blisters. May cause severe allergic reactions.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4542
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979