Madder (Indian madder, Rubia cordifolia) LC
Description
Rubia cordifolia is a small bush plant that can grow to 1.5 m in height. The evergreen leaves are 5–10 cm long and 2–3 cm broad, produced in whorls of 4-7 starlike around the central stem.
Historical importance
Rubia cordifolia was an economically important source of a red pigment in many regions of Asia, Europe and Africa. It was extensively cultivated from antiquity until the mid nineteenth century. The plant's roots contain an organic compound called Alizarin, that gives its red colour to a textile dye known as Rose madder. It was also used as a colorant, especially for paint, that is referred to as Madder lake [1].
Examples:
Summary of results
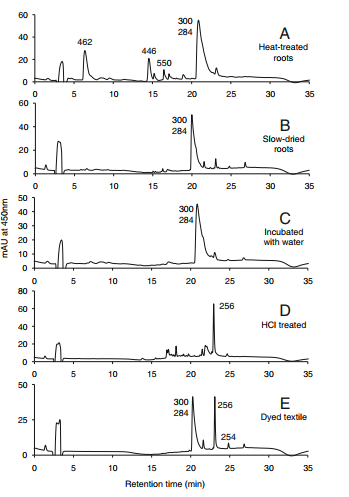
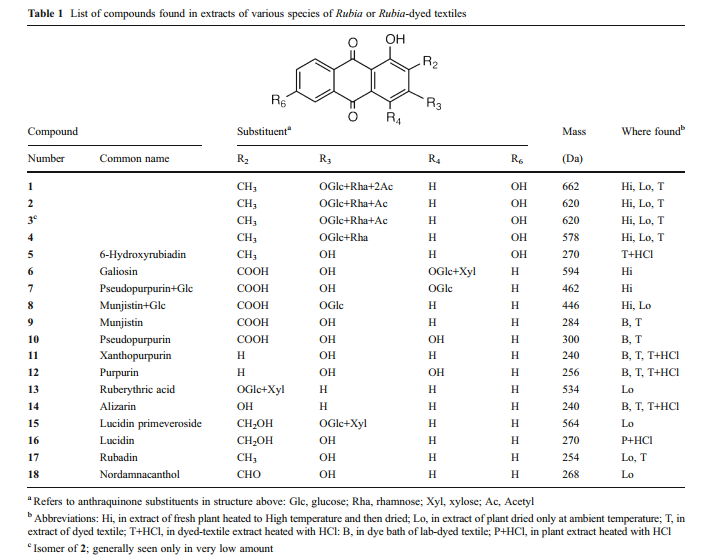
The primary colorants in roots dried at ambient temperature are pseudopurpurin (table 1, compound 10) and munjistin (9) which elute as a single broad, tailing peak in our HPLC system. The reason for the tailing, and occasional changes in shape and elution time, is likely related to the use of dilute formic acid in the HPLC mobile phase. Formic acid is favorable for producing both positive and negative ions in the mass spectrometer, but is not acidic enough to completely suppress ionization of munjistin and pseudopurpurin carboxyl groups. Thus these partially ionized molecules can interact with polar groups on the stationary phase of HPLC column, depending on the degree of end-capping of silanol groups. Nevertheless, pseudopurpurin and munjistin can readily differentiated by ion extraction mass spectrometry [1].
Analytical instrumentation and procedures
Extraction of plant material
Samples of plant specimens were extracted by heating about 10 mg of the ground or chopped, dry plant with 1.0 mL of methanol/water (1:1) at 65 °C for 1 h. The supernatant liquid was removed by pipet and was centrifuged at 12,000 rpm for about 5 min, after which the clear supernatant was subjected to analysis. This solution was diluted with methanol/water (1:1) if necessary.
Extraction of dyed textiles or fibers
Dyed textile or yarn specimens were extracted using a “soft” procedure, namely, by heating approximately 0.1–1 mg of fibers in 200 μL of a solution of pyridine/water/1.0 M oxalic acid in water (95:95:10) at 100 °C for 15 min.
HCl treated
The plant extracts were heated in 2 M HCl at 95 °C for 60 min to hydrolyze O-glycosides or to promote decarboxylation.
Analysis of dye components
Extracts of plant material or of dyed silk or wool were analyzed by HPLC with photodiode array and mass spectrometric detection using an Agilent 1100 high performance liquid chromatography system consisting of an automatic injector, a gradient pump, a Hewlett-Packard series 1100 photodiode array detector, and an Agilent series 1100 VL on-line atmospheric pressure ionization electrospray ionization mass spectrometer. Operation of the system and data analysis were done using ChemStation software, and detection was generally done in the negative ion [M-H]– mode. Separation of dye components was made, in the majority of cases, on a Vydac C18 reversed phase column (2.1 µm dia.×250 µm long; 5-µm particle size). Columns were eluted with acetonitrile-water gradients containing 0.1 % formic acid in both solvents.
Chromatograms
HPLC profiles for Rubia cordifolia. Elution was monitored at 450 nm. The numbers over the peaks are the molecule masses of the principal of otherwise significant components [1].
Sample information
Identified compounds
References
[1] Wikipedia Rubia cordifolia, https://en.wikipedia.org/wiki/Rubia_cordifolia
[2] Chika Mouri and Richard Laursen "Identification of anthraquinone markers for distinguishing Rubia species in madder-dyed textiles by HPLC" Microchimica Acta October 2012, Volume 179, Issue 1–2, pp 105–113