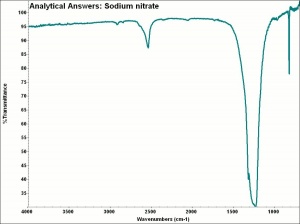
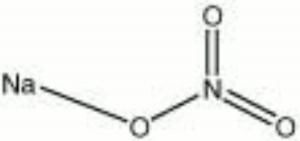
Sodium nitrate
Jump to navigation
Jump to search
Description
Colorless, deliquescent crystals that naturally as Caliche in mineral deposits. Sodium nitrate is primarily used as a fertilizer. It is also used in the manufacture of glass, match heads, and explosives.
Synonyms and Related Terms
caliche; Chili niter; Chile nitre; Chile saltpeter; soda niter; cubic niter; dusiènan sodný (Ces.); Natriumnitrat (Deut.); nitrato sódico (Esp.); nitrate de sodium (Fr.); natriumnitraat (Ned.); azotan(V) sodu (Pol.);
Hazards and Safety
- Toxic by ingestion and inhalation.
- Hygroscopic.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, glycerol. Slightly soluble in ethanol. Crystals are cubic.
| Composition | NaNO3 |
|---|---|
| CAS | 7631-99-4 |
| Melting Point | 308 C |
| Density | 2.267 g/ml |
| Molecular Weight | mol. wt. = 85 |
| Refractive Index | 1.5874, 1.3361 |
| Boiling Point | 380 C (dec) |
Physical and Chemical Properties
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 738
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8792
- Wikipedia: http://en.wikipedia.org/wiki/Sodium_nitrate (Accessed Jan. 15, 2006)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.5874, 1.3361