Ultramarine blue, synthetic
Description
A pure, deep blue, fine particle, synthetic ultramarine discovered in 1826 in France by Jean-Baptiste Guimet and sold commercially in 1828. Synthetic ultramarine is prepared when anhydrous sodium sulfate or Sodium carbonate is mixed with clay, silica, sulfur, rosin and charcoal then slowly heated in a reducing atmosphere to 750º C (1,380º F). Variations in mixture proportions give various shades of blues, greens, violets and reds. Synthetic ultramarine blue has rounded particles that are finer and more regular in size and shape than the natural ultramarine pigment. The synthetic pigment is inexpensive and is used as a permanent artist pigment in oil and watercolor paints. Synthetic ultramarine is also used as a whitener in textiles and paper because in minimized yellowish shades. Additionally, it is used in Wallpaper, Soap, textile printing, and laundry bluing agents.
Synonyms and Related Terms
artificial ultramarine blue; Pigment Blue 29; CI 77007; French ultramarine; Ultramarinblau, synthetisch (Deut.); outremer de synthèse (Fr.); oyltramarina synthetiki (Gr.); blu oltremare artificiale (It.); blu Guimet (It.); ultramar (Esp.); ultramarijn (Ned.); ultramaryna (Pol.); ultramarijn blauw (syn) (Ned.); azul ultramarino, sintético (Port.); French blue; new blue; sky blue; Academy blue; permanent blue; Oriental blue; Gmelins blue; Guimet's blue; royal blue
Risks
- No significant hazards.
- Noncombustible.
Physical and Chemical Properties
- Discolors when exposed to weak acids or sulfur fumes.
- Insoluble in water.
- Small, round uniform particles, no birefringence, no pleochroism, extinct in crossed polars.
- ASTM lightfastness=1 (excellent)
| Composition | 3Na2O.3Al2O3.6SiO2.2Na2S |
|---|---|
| Density | 2.34 g/ml |
| Refractive Index | 1.51; 1.63 |
Comparisons
Characteristics of Common Blue Pigments
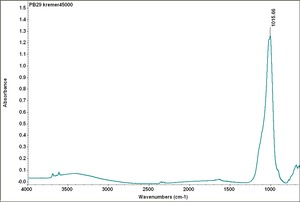
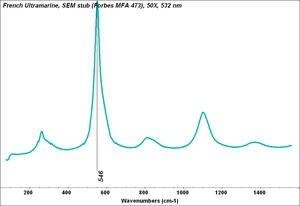
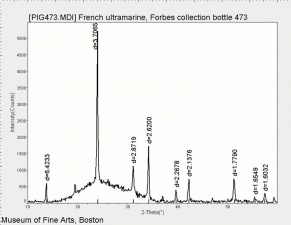
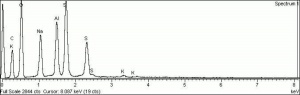
Additional Images
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- J. Plesters, "Ultramarine Blue, Natural and Artificial", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- Pigments through the ages -http://webexhibits.org/pigments/indiv/technical/ultramarine.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- E.J.LaBarre, Dictionary and Encyclopedia of Paper and Paper-making, Swets & Zeitlinger, Amsterdam, 1969
- Encyclopedia Britannica, http://www.britannica.com Comment: "ultramarine." Accessed 17 Mar. 2005 .
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Colour Index International online at www.colour-index.org
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000