Difference between revisions of "Alumina trihydrate"
(username removed) |
|||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white, translucent powder that is also called aluminum hydroxide. Alumina trihydrate is obtained from [ | + | A white, translucent powder that is also called aluminum hydroxide. Alumina trihydrate is obtained from [[bauxite|bauxite]]. When it is strongly heated, alumina trihydrate will convert to [[aluminum%20oxide|aluminum oxide]] with the release of water. Alumina trihydrate is used as a base in the preparation of transparent lake pigments. It is also used as an inert filler in paints and tends to increase the transparency of colors when dispersed in oils. Alumina trihydrate is used commercially as a paper coating, flame retardant, water repellant, and as a filler in glass, ceramics, inks, detergents, cosmetics, and plastics. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | aluminum hydroxide; Pigment White 24; CI 7702; alumina hydrate; | + | aluminum hydroxide; Pigment White 24; CI 7702; alumina hydrate; alúmina trihidratada (Esp.); alumiinivalkoinen (Fin.);alumiinihydrosksidi (Fin.); blanc d'alumine (Fr.); ydroxeidio toy argilioy (Gr.); leyko toy argilioy (aloyminioy) (Gr.); bianco di alluminio (It.); idrossido d'alluminio (It.); aluminium hydroxide (Br.); aluminum trihydrate; hydrated alumina; aluminium trihydrate; hydrated aluminum oxide; aluminum white; aluminum hydrate white; transparent white; gloss white; |
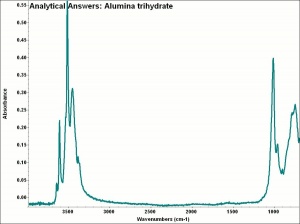
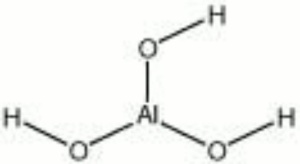
[[[SliderGallery rightalign|aaiAL_TRIHYDRATE.jpg~FTIR|alumina trihydrate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aaiAL_TRIHYDRATE.jpg~FTIR|alumina trihydrate.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| − | + | No significant hazards. | |
| − | |||
| − | |||
| − | + | ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC219130250&productDescription=ALUMINUM+HYDROXIDE+POWDER+25G&catNo=AC219130250&vendorId=VN00032119&storeId=10652 SDS] | |
| + | == Physical and Chemical Properties == | ||
| − | Under plane-polarized light, particles are colorless with low relief | + | * Soluble in mineral acids and caustic soda. Insoluble in water. |
| + | * Fine grains. | ||
| + | * No birefringence. | ||
| + | * Under plane-polarized light, particles are colorless with low relief | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 29: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.42-2.45 | + | | 2.42-2.45 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
| 1.568 - 1.587 | | 1.568 - 1.587 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 45: | Line 42: | ||
[[media:download_file_522.pdf|Characteristics of Common White Pigments]] | [[media:download_file_522.pdf|Characteristics of Common White Pigments]] | ||
| + | == Resources and Citations == | ||
| + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: ref.index =1.50-1.56 | ||
| − | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index: alpha=1.568-1.570; beta=1.568-1.570; gamma 1.586-1.587 | |
| − | |||
| − | * | ||
| − | |||
| − | |||
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * R. Newman, E. Farrell, 'House Paint Pigments', ''Paint in America '', R. Moss ed., Preservation Press, New York City, 1994 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 35 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #355 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #355 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Aluminum." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: Aluminum." Encyclopædia Britannica. 2004. Encyclopædia Britannica Premium Service. 15 Apr. 2004 . |
| − | * Website | + | * Website: http://www.coloria.net/varita.htm - Finnish name |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref.index =1.50-1.56 |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
Latest revision as of 11:00, 26 April 2022
Description
A white, translucent powder that is also called aluminum hydroxide. Alumina trihydrate is obtained from Bauxite. When it is strongly heated, alumina trihydrate will convert to Aluminum oxide with the release of water. Alumina trihydrate is used as a base in the preparation of transparent lake pigments. It is also used as an inert filler in paints and tends to increase the transparency of colors when dispersed in oils. Alumina trihydrate is used commercially as a paper coating, flame retardant, water repellant, and as a filler in glass, ceramics, inks, detergents, cosmetics, and plastics.
Synonyms and Related Terms
aluminum hydroxide; Pigment White 24; CI 7702; alumina hydrate; alúmina trihidratada (Esp.); alumiinivalkoinen (Fin.);alumiinihydrosksidi (Fin.); blanc d'alumine (Fr.); ydroxeidio toy argilioy (Gr.); leyko toy argilioy (aloyminioy) (Gr.); bianco di alluminio (It.); idrossido d'alluminio (It.); aluminium hydroxide (Br.); aluminum trihydrate; hydrated alumina; aluminium trihydrate; hydrated aluminum oxide; aluminum white; aluminum hydrate white; transparent white; gloss white;
Risks
No significant hazards.
ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in mineral acids and caustic soda. Insoluble in water.
- Fine grains.
- No birefringence.
- Under plane-polarized light, particles are colorless with low relief
| Composition | Al(OH)3 |
|---|---|
| CAS | 21645-51-2 |
| Density | 2.42-2.45 g/ml |
| Molecular Weight | mol. wt. = 78.0 |
| Refractive Index | 1.568 - 1.587 |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: ref.index =1.50-1.56
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index: alpha=1.568-1.570; beta=1.568-1.570; gamma 1.586-1.587
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 35
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #355
- Encyclopedia Britannica, http://www.britannica.com Comment: Aluminum." Encyclopædia Britannica. 2004. Encyclopædia Britannica Premium Service. 15 Apr. 2004 .
- Website: http://www.coloria.net/varita.htm - Finnish name
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref.index =1.50-1.56
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000