Difference between revisions of "Baygon"
Jump to navigation
Jump to search
(username removed) |
|||
| (9 intermediate revisions by 3 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | [Johnson] A registered trademark for a series of [ | + | [SC Johnson and Sons] A registered trademark for a series of [[insecticide]] containing either [[propoxur]] or a [[pyrethrin]] derivative originally developed by Bayer in 1975. The active ingredients are still manufactured by Bayer but licensed exclusively to SC Johnson. Over 10 formulations are available for extermination and control of household pests such as crickets, roaches, ants, carpenter ants, spiders, silverfish and mosquitoes. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
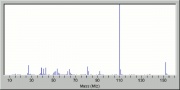
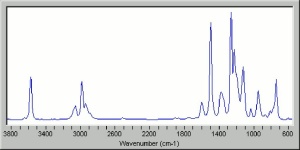
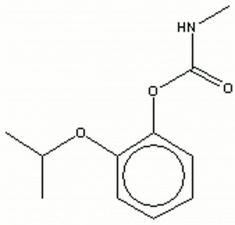
| + | [[[SliderGallery rightalign|propoxurir.jpg~FTIR|propoxurstructure2.jpg~Chemical structure]]] | ||
| + | propoxur; PHC; 2-isopropoxyphenyl methylcarbamate; o-IMPC | ||
| + | == Risks == | ||
| + | |||
| + | * Toxic by ingestion, inhalation and skin absorption. LD50=90-128 mg/kg | ||
| + | * Decomposes to form methyl isocyanate. | ||
| + | * May stain fabrics, plastics, paper and rubber. | ||
| + | * PestWeb: [https://pestweb.ca/assets/files/productdocuments/doc_79FAFFAC4E96DCA1F96594019D1E7C1439EADF99.pdf SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
{| class="wikitable" | {| class="wikitable" | ||
| Line 17: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 91.5 | + | | 91.5 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 23: | Line 31: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * SCJohnson: [https://www.baygon.com.ph/en-ph/products Baygon] | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:03, 2 May 2022
Description
[SC Johnson and Sons] A registered trademark for a series of Insecticide containing either Propoxur or a Pyrethrin derivative originally developed by Bayer in 1975. The active ingredients are still manufactured by Bayer but licensed exclusively to SC Johnson. Over 10 formulations are available for extermination and control of household pests such as crickets, roaches, ants, carpenter ants, spiders, silverfish and mosquitoes.
Synonyms and Related Terms
propoxur; PHC; 2-isopropoxyphenyl methylcarbamate; o-IMPC
Risks
- Toxic by ingestion, inhalation and skin absorption. LD50=90-128 mg/kg
- Decomposes to form methyl isocyanate.
- May stain fabrics, plastics, paper and rubber.
- PestWeb: SDS
Physical and Chemical Properties
| Composition | (CH3)2CHOC6H4OOCNHCH3 |
|---|---|
| CAS | 114-26-1 |
| Melting Point | 91.5 C |
| Molecular Weight | mol. wt. = 209.2 |
Resources and Citations
- SCJohnson: Baygon
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988