Difference between revisions of "Benzaldehyde"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A colorless oil that smells like almonds. Benzaldehyde is primarily used in the organic synthesis of dyes. It is also used as a solvents for [[oil|oils]], [[natural%20resin|natural resins]], as well as some [[cellulose%20ether|cellulose ethers]], and [[cellulose%20ester|cellulose esters]]. Benzaldehyde is found in some photographic chemicals. | A colorless oil that smells like almonds. Benzaldehyde is primarily used in the organic synthesis of dyes. It is also used as a solvents for [[oil|oils]], [[natural%20resin|natural resins]], as well as some [[cellulose%20ether|cellulose ethers]], and [[cellulose%20ester|cellulose esters]]. Benzaldehyde is found in some photographic chemicals. | ||
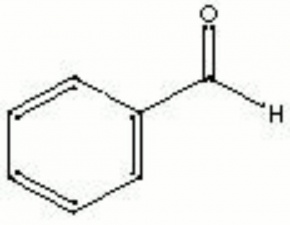
| − | + | [[[SliderGallery rightalign|benzaldehyde.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
benzoic aldehyde; artificial oil of almond; benzenecarbonal | benzoic aldehyde; artificial oil of almond; benzenecarbonal | ||
| − | [ | + | == Hazards and Safety == |
| + | |||
| + | * Skin contact may cause irritation and redness. | ||
| + | * Highly toxic by ingestion. Combustible. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC209790010&productDescription=BENZALDEHYDE+DIMETHYL+AC+1LT&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == Other Properties == | + | ==Physical and Chemical Properties==== Other Properties == |
Miscible with ethanol, ether, oils. Slightly soluble in water. | Miscible with ethanol, ether, oils. Slightly soluble in water. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -56.5 | + | | -56.5 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.0415 | + | | 1.0415 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 179 | + | | 179 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 17:24, 2 May 2022
Description
A colorless oil that smells like almonds. Benzaldehyde is primarily used in the organic synthesis of dyes. It is also used as a solvents for oils, natural resins, as well as some cellulose ethers, and cellulose esters. Benzaldehyde is found in some photographic chemicals.
Synonyms and Related Terms
benzoic aldehyde; artificial oil of almond; benzenecarbonal
Hazards and Safety
- Skin contact may cause irritation and redness.
- Highly toxic by ingestion. Combustible.
- ThermoFisher: SDS
Physical and Chemical Properties==== Other Properties
Miscible with ethanol, ether, oils. Slightly soluble in water.
| Composition | C6H5CHO |
|---|---|
| CAS | 100-52-7 |
| Melting Point | -56.5 C |
| Density | 1.0415 g/ml |
| Molecular Weight | mol. wt. = 106.1 |
| Refractive Index | 1.5440-1.5464 |
| Boiling Point | 179 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1085
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998