Citric acid
Description
Colorless crystals or white powder. Citric acid occurs naturally in many animal and plant cells and is especially concentrated in lemon, lime, and pineapple juice. It was first isolated from plants in 1784 by Karl Scheele. It can also be produced by the fermentation of some sugars. Citric acid is added to beverages, processed fruits, and shampoos as an acidifier, preservative, and foam inhibitor. Citric acid can act as a sequestering agent for some trace metals and has been used to remove iron gall ink and other types of stains from paper. It is also used in medicine, in making blueprint paper, in textile printing, in photographic developers, and as a polishing agent for metals.
Synonyms and Related Terms
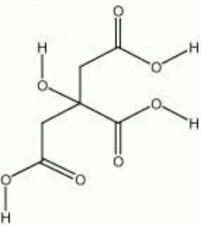
2-hydroxy-1,2,3-propanetricarboxylic acid; 8-hydroxy-tricarballylic acid; Zitronensäure (Deut.); ácido cítrico (Esp.); acide citrique (Fr.); acido citrico (It.); citroenzuur (Ned.); sitronsyre (Nor.); kwas cytrynowy (Pol.); ácido cítrico (Port.); citronsyra (Sven.)
Risks
Combustible. Skin contact with concentrated solutions may cause irritation.
ThermoFisher: SDS
Physical and Chemical Properties
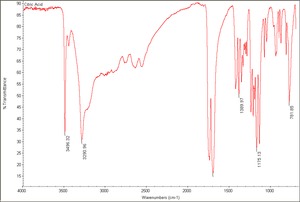
Soluble in water, ethanol, ether. Orthorhombic crystals. pH (0.1 N aqueous solution) = 2.2
| Composition | C6H8O7 |
|---|---|
| CAS | 77-92-9 (Anhydrous) |
| Melting Point | 153 |
| Density | 1.542 |
| Molecular Weight | mol. wt. = 210.14 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Wikipedia: http://en.wikipedia.org/wiki/Citric_acid (Accessed Oct. 18, 2005)
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2387
- Photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH (0.1 N aqueous solution) = 2.2
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998