Difference between revisions of "Lactic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
A thick water-soluble liquid that occurs naturally in sour milk. Lactic acid is also found in fermented molasses, apples, tomato juice, beer, and wine. It is formed by the action of ''Bacillus acidilacti'' on [[glucose]], [[sucrose]], and [[lactose]]. Lactic acid is used in dyeing vats as a mordant for wools and as a solvent for water-insoluble dyes. It is also used in leather processing. | A thick water-soluble liquid that occurs naturally in sour milk. Lactic acid is also found in fermented molasses, apples, tomato juice, beer, and wine. It is formed by the action of ''Bacillus acidilacti'' on [[glucose]], [[sucrose]], and [[lactose]]. Lactic acid is used in dyeing vats as a mordant for wools and as a solvent for water-insoluble dyes. It is also used in leather processing. | ||
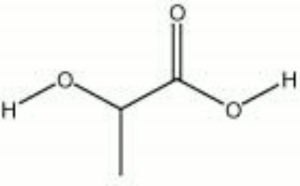
| − | + | [[[SliderGallery rightalign|lactic acid.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
milk acid; alpha-hydroxypropionic acid; DL-lactic acid; 2-hydroxypropanoic acid | milk acid; alpha-hydroxypropionic acid; DL-lactic acid; 2-hydroxypropanoic acid | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Combustible. Flash point = 110 C | ||
| + | * Medium strength acid. | ||
| + | * Contact may cause redness or burns. | ||
| + | * Flinn Scientific: [https://www.flinnsci.com/sds_427-lactic-acid/sds_427/ SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, ethanol, glycerol, furfural. Slightly soluble in ether. Insoluble in chloroform, petroleum ether and carbon disulfide. | Soluble in water, ethanol, glycerol, furfural. Slightly soluble in ether. Insoluble in chloroform, petroleum ether and carbon disulfide. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 18 | + | | 18 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.2 | + | | 1.2 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 122 | + | | 122 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4059 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4059 | ||
Latest revision as of 11:19, 6 October 2022
Description
A thick water-soluble liquid that occurs naturally in sour milk. Lactic acid is also found in fermented molasses, apples, tomato juice, beer, and wine. It is formed by the action of Bacillus acidilacti on Glucose, Sucrose, and Lactose. Lactic acid is used in dyeing vats as a mordant for wools and as a solvent for water-insoluble dyes. It is also used in leather processing.
Synonyms and Related Terms
milk acid; alpha-hydroxypropionic acid; DL-lactic acid; 2-hydroxypropanoic acid
Risks
- Combustible. Flash point = 110 C
- Medium strength acid.
- Contact may cause redness or burns.
- Flinn Scientific: SDS
Physical and Chemical Properties
Soluble in water, ethanol, glycerol, furfural. Slightly soluble in ether. Insoluble in chloroform, petroleum ether and carbon disulfide.
| Composition | CH3CH2OCOOH |
|---|---|
| CAS | 50-21-5 |
| Melting Point | 18 C |
| Density | 1.2 g/ml |
| Molecular Weight | mol. wt. = 90.1 |
| Boiling Point | 122 C |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4059
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985