Difference between revisions of "Methyl silicate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
A colorless, liquid silicate that hydrolyzes to form a colorless, transparent film of [http://cameo.mfa.org/materials/fullrecord.asp?name=silica silica]. Methyl silicate is used as a heat-resistant glass coating. | A colorless, liquid silicate that hydrolyzes to form a colorless, transparent film of [http://cameo.mfa.org/materials/fullrecord.asp?name=silica silica]. Methyl silicate is used as a heat-resistant glass coating. | ||
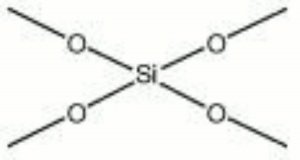
| + | [[[SliderGallery rightalign|methyl silicate.jpg~Chemical structure]]] | ||
| + | == Synonyms and Related Terms == | ||
| − | + | Silicato de metilo (Esp.); silicate de méthyle (Fr.); tetrametil silicato (It.); silicato de metilo (Port.); tetramethoxy silane; silicone ester; tetramethyl silicate; tetramethyl orthosilicate; | |
| − | + | == Risks == | |
| − | [ | + | * Toxic by inhalation and ingestion. |
| + | * Contact can cause redness and burns. | ||
| + | * Flammable. Flash point = 20 C | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC203820250&productDescription=TETRAMETHYL+ORTHOSILICAT+25GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in ethanol. Insoluble in water. | Soluble in ethanol. Insoluble in water. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -2 | + | | -2 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.0232 | + | | 1.0232 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 121 | + | | 121 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:32, 1 October 2022
Description
A colorless, liquid silicate that hydrolyzes to form a colorless, transparent film of silica. Methyl silicate is used as a heat-resistant glass coating.
Synonyms and Related Terms
Silicato de metilo (Esp.); silicate de méthyle (Fr.); tetrametil silicato (It.); silicato de metilo (Port.); tetramethoxy silane; silicone ester; tetramethyl silicate; tetramethyl orthosilicate;
Risks
- Toxic by inhalation and ingestion.
- Contact can cause redness and burns.
- Flammable. Flash point = 20 C
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ethanol. Insoluble in water.
| Composition | (CH3O)4Si |
|---|---|
| CAS | 681-84-5 |
| Melting Point | -2 C |
| Density | 1.0232 g/ml |
| Molecular Weight | mol. wt. = 152.3 |
| Boiling Point | 121 C |
Resources and Citations
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993