Difference between revisions of "Niter"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Naturally occurring mineral composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=potassium | + | Naturally occurring mineral composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=potassium%20nitrate potassium nitrate]. Also known as saltpeter, niter has thin, shiny translucent crystals. It usually occurs as [http://cameo.mfa.org/materials/fullrecord.asp?name=efflorescence efflorescence] on the surface of soils in arid regions. Large quantities of niter have been found in Spain, Italy, Egypt, Arabia, India, Russia, and the United States. Niter was used during the Civil War as a component in [http://cameo.mfa.org/materials/fullrecord.asp?name=gunpowder gunpowder]. It is now used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=glass glass], matches, explosives, and fertilizers. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | potassium nitrate; saltpetre; saltpeter; nitrate of potash; nitre (Br., Fr.); kaliumnitrat (Dan., Deut.); Kalisalpeter (Deut.); | + | potassium nitrate; saltpetre; saltpeter; nitrate of potash; nitre (Br., Fr.); kaliumnitrat (Dan., Deut.); Kalisalpeter (Deut.); salpêtre (Fr.); salpêtre du Chili (Fr.); nitrato di potassio (It.); kaliumnitraat (Ned.); azotan(V) potasu (Pol.); |
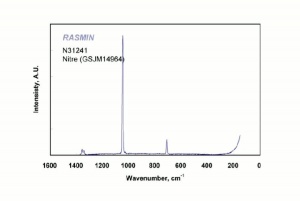
[[[SliderGallery rightalign|nitreRS.jpg~Raman]]] | [[[SliderGallery rightalign|nitreRS.jpg~Raman]]] | ||
| Line 43: | Line 43: | ||
== Authority == | == Authority == | ||
| − | * | + | * R. Mayer, ''The Artist's Handbook of Materials and Techniques'', Viking Press, New York, 1981 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 07:32, 24 July 2013
Description
Naturally occurring mineral composed of potassium nitrate. Also known as saltpeter, niter has thin, shiny translucent crystals. It usually occurs as efflorescence on the surface of soils in arid regions. Large quantities of niter have been found in Spain, Italy, Egypt, Arabia, India, Russia, and the United States. Niter was used during the Civil War as a component in gunpowder. It is now used in the manufacture of glass, matches, explosives, and fertilizers.
Synonyms and Related Terms
potassium nitrate; saltpetre; saltpeter; nitrate of potash; nitre (Br., Fr.); kaliumnitrat (Dan., Deut.); Kalisalpeter (Deut.); salpêtre (Fr.); salpêtre du Chili (Fr.); nitrato di potassio (It.); kaliumnitraat (Ned.); azotan(V) potasu (Pol.);
Other Properties
Soluble in water (38 g in 100 g)
| Composition | KNO3 |
|---|---|
| CAS | 7757-79-1 |
| Melting Point | 334 |
| Density | 2.1 |
| Molecular Weight | 101.1 |
| Boiling Point | 400 (dec) |
Hazards and Safety
Can be mixed with sulfur and charcoal to form gunpowder.
Ingestion can cause nausea and irritation
Authority
- R. Mayer, The Artist's Handbook of Materials and Techniques, Viking Press, New York, 1981
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 632
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7815
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Potassium_nitrate (Accessed Sept. 10, 2005)