Difference between revisions of "Para red"
Jump to navigation
Jump to search
(username removed) |
|||
| (5 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
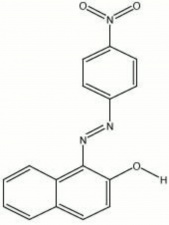
| − | A class of bright cherry-red synthetic organic colorants. Para red was first synthesized in 1880 by Holliday in England. The dye is made by reacting beta-naphthol with [ | + | A class of bright cherry-red synthetic organic colorants. Para red was first synthesized in 1880 by Holliday in England. The dye is made by reacting beta-naphthol with [[paranitroaniline|paranitroaniline]], a coal-tar derivative. Different shades are obtained by varying temperature and pH of the mixture. Para reds have fair lightfastness and are not used in artists paints because of bleeding. They were previously used industrially in metal finishes and printing inks but have been replaced with more durable colors. |
| − | + | [[[SliderGallery rightalign|para red.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
paranitraniline red; Pigment Red 1; CI 12070; p-nitrobenzene-azo-beta-naphthol; Pararot (Deut.); rouge para (Fr.); pararood (Ned.); Signal red; para toner; | paranitraniline red; Pigment Red 1; CI 12070; p-nitrobenzene-azo-beta-naphthol; Pararot (Deut.); rouge para (Fr.); pararood (Ned.); Signal red; para toner; | ||
| − | [ | + | == Risks == |
| + | |||
| + | * Ingestion can cause cyanosis. | ||
| + | * Inhalation and contact may cause irritation. | ||
| + | * Suspected carcinogen and mutagen. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/09522.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
Insoluble in water and ethanol. | Insoluble in water and ethanol. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 248-252 | + | | 248-252 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 33: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: first made by Mssrs Holliday and Sons in England in 1880 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ''ICOM-CC Preprints'' Lyon, Getty Conservation Institute, Los Angeles, 1999 Comment: first made in 1880 by Holliday |
| − | * | + | * B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', ''Studies in the History of Art'' , National Gallery of Art, Washington DC, No. 57, 1997 |
| − | * | + | * M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ''ICOM Preprints'' Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999 Comment: Gallois and Ullrich in 1885 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Monona Rossol, ''The Artist's Complete Health and Safety Guide'', Allworth Press, New York, 1994 |
* Colour Index International online at www.colour-index.org | * Colour Index International online at www.colour-index.org | ||
| Line 62: | Line 55: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * Website | + | * Website: www.straw.com/sig/dyehist - first made in 1885 by von Gallois and Ullrich |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:53, 23 August 2022
Description
A class of bright cherry-red synthetic organic colorants. Para red was first synthesized in 1880 by Holliday in England. The dye is made by reacting beta-naphthol with Paranitroaniline, a coal-tar derivative. Different shades are obtained by varying temperature and pH of the mixture. Para reds have fair lightfastness and are not used in artists paints because of bleeding. They were previously used industrially in metal finishes and printing inks but have been replaced with more durable colors.
Synonyms and Related Terms
paranitraniline red; Pigment Red 1; CI 12070; p-nitrobenzene-azo-beta-naphthol; Pararot (Deut.); rouge para (Fr.); pararood (Ned.); Signal red; para toner;
Risks
- Ingestion can cause cyanosis.
- Inhalation and contact may cause irritation.
- Suspected carcinogen and mutagen.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Insoluble in water and ethanol.
| Composition | C16H11N3O3 |
|---|---|
| CAS | 6410-10-2 |
| Melting Point | 248-252 C |
| Molecular Weight | mol. wt.=293.27 |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: first made by Mssrs Holliday and Sons in England in 1880
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999 Comment: first made in 1880 by Holliday
- B. Berrie, S.Q. Lomax, 'Azo Pigments: Their History, Synthesis, Properties and Use in Artists' Materials', Studies in the History of Art , National Gallery of Art, Washington DC, No. 57, 1997
- M. de Keijzer, 'A survey of red and yellow modern synthetic organic artists pigments discovered in the 20th century and used in oil colors', ICOM Preprints Lyons, France, Getty Conservation Institute, Los Angeles, p. 369, 1999 Comment: Gallois and Ullrich in 1885
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- Colour Index International online at www.colour-index.org
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website: www.straw.com/sig/dyehist - first made in 1885 by von Gallois and Ullrich