Difference between revisions of "Quinacrine dihydrochloride"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
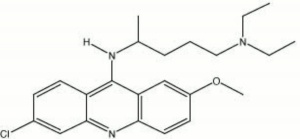
| − | A bright yellow [http://cameo.mfa.org/materials/fullrecord.asp?name=fluorochrome fluorochrome] powder. Quinacrine dihydrochloride dissolves in hot water to produce a solution that is vividly fluorescent under [http://cameo.mfa.org/materials/fullrecord.asp?name=ultraviolet | + | A bright yellow [http://cameo.mfa.org/materials/fullrecord.asp?name=fluorochrome fluorochrome] powder. Quinacrine dihydrochloride dissolves in hot water to produce a solution that is vividly fluorescent under [http://cameo.mfa.org/materials/fullrecord.asp?name=ultraviolet%20radiation ultraviolet light]. The mean excitation wavelength for quinacrine dihydrochloride is 440 nm and the mean emission wavelength is 510 nm (Wolbers, et al, 1990). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 40: | Line 40: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 199 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, ''Notes for Workshop on New Methods in the Cleaning of Paintings'', J.Paul Getty Trust, Los Angeles, 1990 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8225 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8225 | ||
Revision as of 07:33, 24 July 2013
Description
A bright yellow fluorochrome powder. Quinacrine dihydrochloride dissolves in hot water to produce a solution that is vividly fluorescent under ultraviolet light. The mean excitation wavelength for quinacrine dihydrochloride is 440 nm and the mean emission wavelength is 510 nm (Wolbers, et al, 1990).
Synonyms and Related Terms
quinacrine mustard; quinacrine dihydrochloride dihydrate; Atabrine hydrochloride; RP-866; SN-390; mepacrine
Other Properties
Soluble in hot water (pH = 4.5 for 1% solution). Slightly soluble in cold water, ethanol and methanol. Insoluble in ether, benzene, acetone.
| Composition | C23H30ClN3O-2HCl-2H2O |
|---|---|
| CAS | 69-05-6 |
| Melting Point | 250 (dec) |
| Molecular Weight | mol. wt. = 508.6293 |
Hazards and Safety
Toxic. Light sensitive. Hygroscopic.
Fisher Scientific: MSDS
Additional Information
R. Wolbers, N. Sterman, C. Stavroudis, "Notes for Workshop on New Methods in the Cleaning of Paintings", Getty Conservation Institute, Los Angeles, 1990.
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 199
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Richard C. Wolbers, Nanette T. Sterman, Chris Stavroudis, Notes for Workshop on New Methods in the Cleaning of Paintings, J.Paul Getty Trust, Los Angeles, 1990
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8225
- Aldrich Chemical Catalog