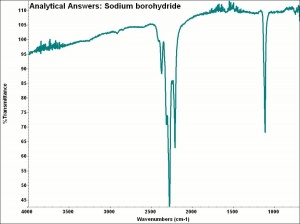
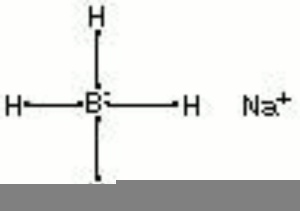
Sodium borohydride
Description
White, crystalline powder. Sodium borohydride reacts with many compounds as a reducing agent and an antichlor. It was introduced in the 1970s for use as a weak bleach for wood pulp, textile stains, and foxing spots (concentrations 0.01-1%). Sodium borohydride is used industrially as a scavenger for aldehydes, ketones, acids, esters, chlorides, disulfides, and nitriles in organic solutions.
Synonyms and Related Terms
sodium tetrahydroborate; sodium hydroboride
Other Properties
Soluble in water, ethanol, ammonia, amines, pyridine. Insoluble in hydrocarbons, alkyl chloride.
| Composition | NaBH4 |
|---|---|
| CAS | 16940-66-2 |
| Melting Point | 36 |
| Density | 1.07 |
| Molecular Weight | mol. wt. = 37.83 |
| Boiling Point | 400 |
Hazards and Safety
Reacts with water to produce hydrogen (highly flammable) and sodium hydroxide.
Hygroscopic. Slowly decomposes in moist air.
Flammable. Dangerous fire risk. May be explosive.
Contact, inhalation, and ingestion results in severe irritation and tissue burns.
Mallinckrodt Baker: MSDS
Additional Information
° S.Adler, "Borohydride: An Alternative to Oxidative Bleaching of Cellulosic Textiles" in Textile Specialty Group Postprints, AIC meeting, 1998. ° I.Block, A.M.Roy, "Treatment of Cellulosic Textiles with Sodium Borohydride" ICOM Preprints, Eighth Triennial Meeting, Sydney Australia, 345-351.
Sources Checked for Data in Record
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.345
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8735
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986