Difference between revisions of "Sodium formaldehydesulfoxylate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 2: | Line 2: | ||
A white, foul-smelling, solid material used as a [[bleaching%20agent|bleaching agent]] for [[textile|textiles]], [[soap|soap]], and molasses. Sodium formaldehydesulfoxylate is also used in vat color printing pastes. | A white, foul-smelling, solid material used as a [[bleaching%20agent|bleaching agent]] for [[textile|textiles]], [[soap|soap]], and molasses. Sodium formaldehydesulfoxylate is also used in vat color printing pastes. | ||
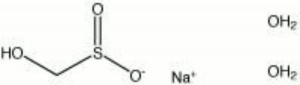
| − | + | [[[SliderGallery rightalign|sodium formaldehydesulfoxylate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
sodium formaldehydesulfoxylate dihydrate; formaldehyde sodium sulfoxylate; hydroxymethanesulfinic acid sodium salt; sodium sulfoxylate; sodium hydroxymethanesulfinate; Rongalite; Rongalite C | sodium formaldehydesulfoxylate dihydrate; formaldehyde sodium sulfoxylate; hydroxymethanesulfinic acid sodium salt; sodium sulfoxylate; sodium hydroxymethanesulfinate; Rongalite; Rongalite C | ||
| − | + | == Hazards and Safety == | |
| − | |||
| − | == | ||
| − | + | * Releases toxic formaldehyde fumes. | |
| + | * May discolorize some blues and greens. | ||
| + | * Contact, ingestion, and inhalation causes irritation. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/87384.htm MSDS] | ||
| − | + | == Physical and Chemical Properties == | |
| − | Hygroscopic. | + | * Soluble in water. Insoluble in absolute ethanol, ether, benzene. |
| + | * Decomposed by acids. | ||
| + | * Hygroscopic. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 63-64 | + | | 63-64 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 32: | Line 35: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 08:13, 2 June 2022
Description
A white, foul-smelling, solid material used as a Bleaching agent for textiles, Soap, and molasses. Sodium formaldehydesulfoxylate is also used in vat color printing pastes.
Synonyms and Related Terms
sodium formaldehydesulfoxylate dihydrate; formaldehyde sodium sulfoxylate; hydroxymethanesulfinic acid sodium salt; sodium sulfoxylate; sodium hydroxymethanesulfinate; Rongalite; Rongalite C
Hazards and Safety
- Releases toxic formaldehyde fumes.
- May discolorize some blues and greens.
- Contact, ingestion, and inhalation causes irritation.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Soluble in water. Insoluble in absolute ethanol, ether, benzene.
- Decomposed by acids.
- Hygroscopic.
| Composition | NaHSO2-HCHO-2H2O |
|---|---|
| CAS | 6035-47-8 |
| Melting Point | 63-64 C |
| Molecular Weight | mol. wt. = 154.11 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8764