Difference between revisions of "Realgar"
(username removed) |
|||
| (13 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:212 realgar.jpg|thumb|Realgar]] | [[File:212 realgar.jpg|thumb|Realgar]] | ||
== Description == | == Description == | ||
| − | + | [[File:realgar C100x.jpg|thumb|realgar at 100x (visible light left; UV light right)]] | |
| − | A bright orange-red mineral composed of [ | + | A bright orange-red mineral composed of [[arsenic%20disulfide|arsenic disulfide]]. Realgar occurs naturally in the Czech Republic, Romania, Macedonia, China, Japan, Peru and the United States (Utah, Nevada, Wyoming, California) in [[gold]], [[silver]], and [[lead]] ores along with [[orpiment]] (arsenic trisulfide). Realgar was once widely used as a pigment because of its bright rich color, but perhaps less so than its mineral congener, orpiment. Early occurrences are known for works of art from China, India, Central Asia, and Egypt. In European painting, apart from fairly regular use in Venice in the 16th-century, the pigment occurs occasionally until about the middle of the 18th-century. It is a usual choice for the bright orange flowers depicted in Dutch 17th-century paintings, and enjoyed moderately regular use in British 17th- century and 18th-century painting, including as a pastel color. However, it is extremely toxic, which has had an effect on its range of application and availability. Realgar is not particularly stable and can undergo a transformation to a yellow polymorph, [[pararealgar]], As4S4; it can also deteriorate badly in oil paint films, resulting in rupturing, cracking and chalking, the pigment itself can fade under the action of light. Arsenic disulfide was made synthetically in the 18th century and sold as arsenic orange. The synthetic variety was purer and less expensive, but is no longer used because of its toxicity. |
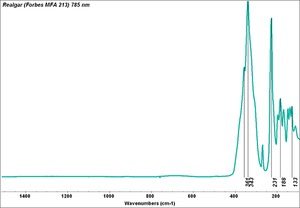
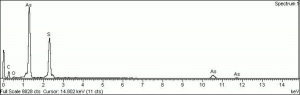
| − | + | [[[SliderGallery rightalign|Realgar (Forbes MFA 213) 785 nm resize.tif~Raman (MFA)|f212sem.jpg~SEM|f212edsbw.jpg~EDS]]] | |
| − | [[ | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | arsenic disulfide; Pigment Yellow 39; CI 77085; realgar (Eng., Esp., Gr., It., Ned., Port.); Realgar (Deut.); rejalgar (Esp.); | + | arsenic disulfide; Pigment Yellow 39; CI 77085; realgar (Eng., Esp., Gr., It., Ned., Port.); Realgar (Deut.); rejalgar (Esp.); réalgar (Fr.); yuo (Jap.); Schwefelarsenik (Deut.); Sandarack (Deut.); Rauschrot (Deut.); red arsenic sulfide; arsenic orange; red orpiment; burnt orpiment; ruby sulfur; risalgallo; oropimente quemado; jalde; roseaker |
| − | |||
| − | |||
| − | == | + | == Risks == |
| − | + | * Turns black in contact with copper and lead containing pigments. | |
| + | * Toxic by inhalation and ingestion. | ||
| + | * Carcinogenic | ||
| − | + | ==Physical and Chemical Properties== | |
| − | Fracture = conchoidal. Luster = resinous to adamantine. Streak = orange-yellow | + | * Isotropic crystals with good cleavage in one direction. |
| + | * Burns with blue flame. | ||
| + | * Darkens with heat. | ||
| + | * Changes to red crystalline form at 170C. | ||
| + | * Soluble in alkaline sulfide solutions and nitric acid. Insoluble in water and hydrochloric acid. | ||
| + | * Fracture = conchoidal. | ||
| + | * Luster = resinous to adamantine. | ||
| + | * Streak = orange-yellow | ||
Pigment particles are pleochroic with high birefringence and straight extinction | Pigment particles are pleochroic with high birefringence and straight extinction | ||
| Line 30: | Line 36: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 307-320 | + | | 307-320 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.56-3.59 | + | | 3.56-3.59 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 39: | Line 45: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 565 | + | | 565 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | V | |
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Realgar Realgar] Accessed Setp. 14, 2005 and August 2023 | |
| − | + | * E.West FitzHugh, "Orpiment and Realgar", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Realgar.shtml Realgar]Record content reviewed by EU-Artech, November 2007. | |
| − | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | |
| − | + | * External source or communication Comment: Submitted information: Ashok Roy, November 2007 | |
| − | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 69 | |
| − | E.West FitzHugh, "Orpiment and Realgar", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Realgar." Accessed 12 May 2004 . |
| − | |||
| − | |||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Realgar." | ||
| − | |||
* ''Artists' Pigments: A Handbook of their History and Characteristics'', Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: E.West FitzHugh, "Orpiment and Realgar" | * ''Artists' Pigments: A Handbook of their History and Characteristics'', Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: E.West FitzHugh, "Orpiment and Realgar" | ||
| − | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 3.56 and ref. index = 2.46; 2.61; 2.59 | |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 |
| − | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | |
| − | * | + | * Pigments Through the Ages -http://webexhibits.org/pigments/indiv/technical/realgar.html - Refractive index: alpha = 2.4; beta = 2.81; gamma =3.02 |
| − | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | |
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | * | ||
| − | |||
| − | |||
| − | |||
| − | * Art and Architecture Thesaurus Online, | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:58, 29 February 2024
Description
A bright orange-red mineral composed of Arsenic disulfide. Realgar occurs naturally in the Czech Republic, Romania, Macedonia, China, Japan, Peru and the United States (Utah, Nevada, Wyoming, California) in Gold, Silver, and Lead ores along with Orpiment (arsenic trisulfide). Realgar was once widely used as a pigment because of its bright rich color, but perhaps less so than its mineral congener, orpiment. Early occurrences are known for works of art from China, India, Central Asia, and Egypt. In European painting, apart from fairly regular use in Venice in the 16th-century, the pigment occurs occasionally until about the middle of the 18th-century. It is a usual choice for the bright orange flowers depicted in Dutch 17th-century paintings, and enjoyed moderately regular use in British 17th- century and 18th-century painting, including as a pastel color. However, it is extremely toxic, which has had an effect on its range of application and availability. Realgar is not particularly stable and can undergo a transformation to a yellow polymorph, Pararealgar, As4S4; it can also deteriorate badly in oil paint films, resulting in rupturing, cracking and chalking, the pigment itself can fade under the action of light. Arsenic disulfide was made synthetically in the 18th century and sold as arsenic orange. The synthetic variety was purer and less expensive, but is no longer used because of its toxicity.
Synonyms and Related Terms
arsenic disulfide; Pigment Yellow 39; CI 77085; realgar (Eng., Esp., Gr., It., Ned., Port.); Realgar (Deut.); rejalgar (Esp.); réalgar (Fr.); yuo (Jap.); Schwefelarsenik (Deut.); Sandarack (Deut.); Rauschrot (Deut.); red arsenic sulfide; arsenic orange; red orpiment; burnt orpiment; ruby sulfur; risalgallo; oropimente quemado; jalde; roseaker
Risks
- Turns black in contact with copper and lead containing pigments.
- Toxic by inhalation and ingestion.
- Carcinogenic
Physical and Chemical Properties
- Isotropic crystals with good cleavage in one direction.
- Burns with blue flame.
- Darkens with heat.
- Changes to red crystalline form at 170C.
- Soluble in alkaline sulfide solutions and nitric acid. Insoluble in water and hydrochloric acid.
- Fracture = conchoidal.
- Luster = resinous to adamantine.
- Streak = orange-yellow
Pigment particles are pleochroic with high birefringence and straight extinction
| Composition | AsS or As4S4 |
|---|---|
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 307-320 C |
| Density | 3.56-3.59 g/ml |
| Refractive Index | 2.538, 2.684, 2.704 |
| Boiling Point | 565 C |
Resources and Citations
V
- Wikipedia: Realgar Accessed Setp. 14, 2005 and August 2023
- E.West FitzHugh, "Orpiment and Realgar", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- Mineralogy Database: RealgarRecord content reviewed by EU-Artech, November 2007.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- External source or communication Comment: Submitted information: Ashok Roy, November 2007
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 69
- Encyclopedia Britannica, http://www.britannica.com Comment: "Realgar." Accessed 12 May 2004 .
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: E.West FitzHugh, "Orpiment and Realgar"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 3.56 and ref. index = 2.46; 2.61; 2.59
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Pigments Through the Ages -http://webexhibits.org/pigments/indiv/technical/realgar.html - Refractive index: alpha = 2.4; beta = 2.81; gamma =3.02
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000