Difference between revisions of "Tetrachloroethane"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A clear, sweet-smelling, viscous liquid. Tetrachloroethane was formerly used as a solvent for [[fat|fats]], [[oil|oils]], [[wax|waxes]], [[natural%20resin|resins]], [[plastic|plastics]], [[cellulose%20acetate|cellulose acetate]], [[rubber | + | A clear, sweet-smelling, viscous liquid. Tetrachloroethane was formerly used as a solvent for [[fat|fats]], [[oil|oils]], [[wax|waxes]], [[natural%20resin|resins]], [[plastic|plastics]], [[cellulose%20acetate|cellulose acetate]], [[rubber|rubber]], [[copal|copal]], and [[sulfur|sulfur]]. It was also used in the manufacture of paint and varnish [[paint%20remover|removers]] and in the production of [[insecticide|insecticides]], [[herbicide|weed killers]], and [[fumigant|fumigants]]. Tetrachlorethane is suspected to be a carcinogen. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
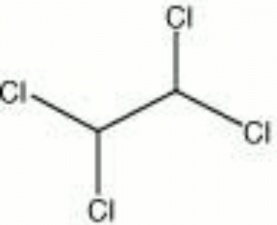
[[[SliderGallery rightalign|tetrachloroethane.jpg~Chemical structure]]] | [[[SliderGallery rightalign|tetrachloroethane.jpg~Chemical structure]]] | ||
| + | == Risks == | ||
| + | |||
| + | Nonflammable. Decomposed on contact with flame or UV light to form toxic fumes (phosgene and hydrogen chloride). | ||
| + | |||
| + | Potential carcinogenic. Toxic by ingestion, inhalation and skin absorption. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC147940010&productDescription=1%2C1%2C2%2C2-TETRACHLOROETHAN+1LT&catNo=AC147940010&vendorId=VN00032119&storeId=10652 SDS] | ||
== Other Properties == | == Other Properties == | ||
| Line 37: | Line 44: | ||
|} | |} | ||
| − | == | + | == Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 805 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 805 | ||
Revision as of 16:34, 4 August 2020
Description
A clear, sweet-smelling, viscous liquid. Tetrachloroethane was formerly used as a solvent for fats, oils, waxes, resins, plastics, Cellulose acetate, Rubber, Copal, and Sulfur. It was also used in the manufacture of paint and varnish removers and in the production of insecticides, weed killers, and fumigants. Tetrachlorethane is suspected to be a carcinogen.
Synonyms and Related Terms
acetylene tetrachloride; sym-tetrachloroethane; Cellon; Bonoform
Risks
Nonflammable. Decomposed on contact with flame or UV light to form toxic fumes (phosgene and hydrogen chloride).
Potential carcinogenic. Toxic by ingestion, inhalation and skin absorption.
ThermoFisher: SDS
Other Properties
Miscible with methanol, ethanol, benzene, ether, carbon tetrachloride, chloroform carbon disulfide, dimethylformamide, oils. Slightly soluble in water.
| Composition | CHCl2CHCl2 |
|---|---|
| CAS | 79-34-5 |
| Melting Point | -44 |
| Density | 1.587-1.593 |
| Molecular Weight | mol. wt. = 167.9 |
| Refractive Index | 1.49419 |
| Boiling Point | 146.5 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 805
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9331