Difference between revisions of "Kraft process"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
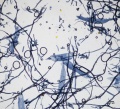
[[File:SUBK 10x2 label.jpg|thumb|Unbleached softwood kraft fibers stained with Graff "C" Stain]] | [[File:SUBK 10x2 label.jpg|thumb|Unbleached softwood kraft fibers stained with Graff "C" Stain]] | ||
== Description == | == Description == | ||
| − | A chemical method of producing paper pulp from wood using [[sodium hydroxide]] and [[sodium sulfide]] | + | A chemical method of producing paper pulp from wood chips using [[sodium hydroxide]] and [[sodium sulfide]] in hot water. The kraft process has been used since 1890, but only gained popularity after an efficient system developed in 1930s to recover and reuse the inorganic pulping chemicals. Now, the kraft process is the most popular method for producing wood pulp in the US due to advantages over [[soda process|soda]] and [[sulfite process|sulfite]] pulping methods. Unlike the sulfite process, any wood species can be used. Additionally, cooking times are reduced, and the waste recovery is nearly a closed cycle process. Chemically, the kraft process breaks the bonds that link [[lignin]], [[hemicellulose]] and [[cellulose]], then breaks down the lignin and hemicellulose into soluble fragments while leaving the cellulose fibers. In the final recovery process, [[rosin]] soaps, [[tall oil]], and [[Turpentine (oil)|Turpentine]] are by-products as well as volatile sulfur emissions. The resultant [[kraft paper]] is strong but brown due to approximately 5% residual lignin. Bleaching can be done to produce white paper, but this also decreases the strength of the fibers. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
== Other Properties == | == Other Properties == | ||
| − | Kraft pulp tends to be stronger than other types of pulp, but actual strength varies by species. Due to higher levels of | + | Kraft pulp tends to be stronger than other types of pulp, but actual strength varies by species. Due to higher levels of chromophores, kraft pulps tend to be darker than sulfite pulps. |
When stained with [[Graff "C" stain]], kraft pulp can appear in a range of colors depending on the wood type (softwood or hardwood) and the amount of bleaching. Unbleached kraft pulps will appear yellow or blue/grey. With increased bleaching, pulps will appear lighter and tend more toward red when treated with stain, though this can be difficult to see with hardwood pulps. The stained color of kraft pulp tends to be darker than [[sulfite process|sulfite]] pulp. | When stained with [[Graff "C" stain]], kraft pulp can appear in a range of colors depending on the wood type (softwood or hardwood) and the amount of bleaching. Unbleached kraft pulps will appear yellow or blue/grey. With increased bleaching, pulps will appear lighter and tend more toward red when treated with stain, though this can be difficult to see with hardwood pulps. The stained color of kraft pulp tends to be darker than [[sulfite process|sulfite]] pulp. | ||
| Line 21: | Line 21: | ||
</gallery> | </gallery> | ||
| − | == | + | ==Resources and Citations== |
* J.R.G. Bryce.“Sulfite Pulping” and “Alkaline Pulping”. ''Pulp and Paper: Chemistry and Chemical Technology, Volume 1, Edition 3.'' John Wiley & Sons, 1980. James P. Casey Ed. | * J.R.G. Bryce.“Sulfite Pulping” and “Alkaline Pulping”. ''Pulp and Paper: Chemistry and Chemical Technology, Volume 1, Edition 3.'' John Wiley & Sons, 1980. James P. Casey Ed. | ||
| − | |||
* Christopher Biermann. ''Essentials of Pulping and Papermaking''. Academic Press, 1993. | * Christopher Biermann. ''Essentials of Pulping and Papermaking''. Academic Press, 1993. | ||
| − | |||
* J. H. Graff "Color Atlas for Fiber Identification" The Institute of Paper Chemistry, Appleton, WI, 1940. | * J. H. Graff "Color Atlas for Fiber Identification" The Institute of Paper Chemistry, Appleton, WI, 1940. | ||
| − | |||
* Walter Rantanen. 'Fiber ID Course.' Integrated Paper Services. June 2013. Lecture. | * Walter Rantanen. 'Fiber ID Course.' Integrated Paper Services. June 2013. Lecture. | ||
| + | * TAPPI Official Standard T401 om-08. ''Fiber analysis of paper and paperboard''. 2008. http://www.tappi.org/Downloads/Test-Methods/UNTITLED-0104T401pdf.aspx | ||
| + | * Wikipedia: [https://en.wikipedia.org/wiki/Kraft_process Kraft process] Accessed Nov. 2024 | ||
| − | + | [[Category:Materials database]][[Category:MWG]][[Category:Sheet, Cellulose]] | |
Latest revision as of 09:51, 12 November 2024
Description
A chemical method of producing paper pulp from wood chips using Sodium hydroxide and Sodium sulfide in hot water. The kraft process has been used since 1890, but only gained popularity after an efficient system developed in 1930s to recover and reuse the inorganic pulping chemicals. Now, the kraft process is the most popular method for producing wood pulp in the US due to advantages over soda and sulfite pulping methods. Unlike the sulfite process, any wood species can be used. Additionally, cooking times are reduced, and the waste recovery is nearly a closed cycle process. Chemically, the kraft process breaks the bonds that link Lignin, Hemicellulose and Cellulose, then breaks down the lignin and hemicellulose into soluble fragments while leaving the cellulose fibers. In the final recovery process, Rosin soaps, Tall oil, and Turpentine are by-products as well as volatile sulfur emissions. The resultant Kraft paper is strong but brown due to approximately 5% residual lignin. Bleaching can be done to produce white paper, but this also decreases the strength of the fibers.
Synonyms and Related Terms
Sulfate pulp; kraft pulping; alkaline pulping; kraft pulp; softwood bleached kraft; softwood kraft; hardwood bleached kraft; hardwood kraft
Other Properties
Kraft pulp tends to be stronger than other types of pulp, but actual strength varies by species. Due to higher levels of chromophores, kraft pulps tend to be darker than sulfite pulps.
When stained with Graff "C" stain, kraft pulp can appear in a range of colors depending on the wood type (softwood or hardwood) and the amount of bleaching. Unbleached kraft pulps will appear yellow or blue/grey. With increased bleaching, pulps will appear lighter and tend more toward red when treated with stain, though this can be difficult to see with hardwood pulps. The stained color of kraft pulp tends to be darker than sulfite pulp.
Additional Images
Resources and Citations
- J.R.G. Bryce.“Sulfite Pulping” and “Alkaline Pulping”. Pulp and Paper: Chemistry and Chemical Technology, Volume 1, Edition 3. John Wiley & Sons, 1980. James P. Casey Ed.
- Christopher Biermann. Essentials of Pulping and Papermaking. Academic Press, 1993.
- J. H. Graff "Color Atlas for Fiber Identification" The Institute of Paper Chemistry, Appleton, WI, 1940.
- Walter Rantanen. 'Fiber ID Course.' Integrated Paper Services. June 2013. Lecture.
- TAPPI Official Standard T401 om-08. Fiber analysis of paper and paperboard. 2008. http://www.tappi.org/Downloads/Test-Methods/UNTITLED-0104T401pdf.aspx
- Wikipedia: Kraft process Accessed Nov. 2024