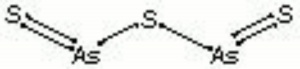Difference between revisions of "Arsenic trisulfide"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:orpimentdw.jpg|thumb|Orpiment crystals]] | [[File:orpimentdw.jpg|thumb|Orpiment crystals]] | ||
| + | [[File:SC282225-pt.jpg|thumb|Hokusai from series of Perspective Pictures <br>MFA# 11.17572 (yellow is orpiment)]] | ||
| + | [[File:orpiment C100x.jpg|thumb|orpiment]] | ||
== Description == | == Description == | ||
| + | [[File:333334 orpiment_2up.jpg|thumb|Orpiment, powdered]] | ||
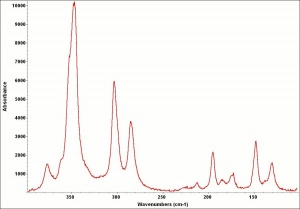
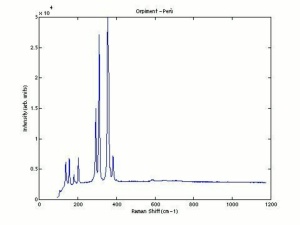
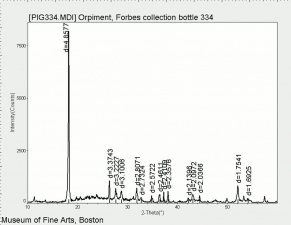
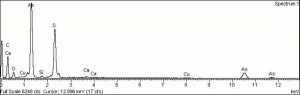
| + | [[[SliderGallery rightalign|OrpimentUCL.jpg~Raman|Orpimentitaly1.jpg~Raman|PIG334.jpg~XRD|f334sem.jpg~SEM|f334edsbw.jpg~EDS|arsenic trisulfide.jpg~Chemical structure]]] | ||
A bright yellow, crystalline powder that occurs naturally as the mineral [[orpiment|orpiment]]. Orpiment is often found with lead and silver ores along with [[realgar|realgar]] (arsenic disulfide). It was once widely used as a pigment because of its bright rich color. However, it is extremely toxic which has limited its use and availability. Arsenic trisulfide was also used to dehair and tan leathers, to kill rodents, and to print calico textiles. It is still used in fireworks and in the manufacture of infrared transmitting glass. | A bright yellow, crystalline powder that occurs naturally as the mineral [[orpiment|orpiment]]. Orpiment is often found with lead and silver ores along with [[realgar|realgar]] (arsenic disulfide). It was once widely used as a pigment because of its bright rich color. However, it is extremely toxic which has limited its use and availability. Arsenic trisulfide was also used to dehair and tan leathers, to kill rodents, and to print calico textiles. It is still used in fireworks and in the manufacture of infrared transmitting glass. | ||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | |||
arsenic (III) sulfide; Pigment Yellow 39; CI 77085, 77086; orpiment (mineral); arsenic sulphide (Br.); Operment (Deut.); Rauschgelb (Deut.); Konigsgelb (Deut.); jaune royal (Fr.); trisulfure d'arsenic (Fr.); oropimente (Esp.); orpimento (It.); trisolfuro d'arsenico (It.); kio (Jap.); sekio (Jap.); ouropigmento (Port.); yellow arsenic sulfide; king's yellow; arsenic yellow; jaune royal; king's gold; arsenous sulfide; arsenic tersulfide; arsenious sulfide; auripigment | arsenic (III) sulfide; Pigment Yellow 39; CI 77085, 77086; orpiment (mineral); arsenic sulphide (Br.); Operment (Deut.); Rauschgelb (Deut.); Konigsgelb (Deut.); jaune royal (Fr.); trisulfure d'arsenic (Fr.); oropimente (Esp.); orpimento (It.); trisolfuro d'arsenico (It.); kio (Jap.); sekio (Jap.); ouropigmento (Port.); yellow arsenic sulfide; king's yellow; arsenic yellow; jaune royal; king's gold; arsenous sulfide; arsenic tersulfide; arsenious sulfide; auripigment | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Highly toxic by ingestion and inhalation. Skin contact causes irritation. Carcinogen and mutagen. | + | * Highly toxic by ingestion and inhalation. |
| + | * Skin contact causes irritation. | ||
| + | * Carcinogen and mutagen. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/84980.htm MSDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| + | == Physical and Chemical Properties == | ||
| + | * Composition = As2S3 (mol. wt. = 246.04) | ||
| + | * CAS = 1303-33-9 | ||
| + | * Melting Point = 310 C | ||
| + | * Mohs Hardness = 1.5 - 2.0 | ||
| + | * Density = 3.43 g/ml | ||
| + | * Refractive Index = 2.40; 2.81; 3.02 | ||
| + | * Boiling Point = 707 C | ||
| + | * Soluble in nitric acid, alkali sulfide and ammonia solutions. Insoluble in water and hydrochloric acid. | ||
| + | * Unstable when mixed with alkaline pigments such as in buon fresco or in combinations with lime white | ||
| + | * Oxidizes to form translucent or white oxides of arsenic. | ||
| + | * Both orpiment and realgar lose color on exposure to light | ||
| + | * Small, often elongated particles (4-20 microns); | ||
| + | * Strong yellow particles with high birefringence and straight extinction | ||
| + | * Luster = pearly to resinous. | ||
| + | * Streak = lemon-yellow. | ||
| − | + | In PPL, orpiment has a strong yellow color and exhibits very high relief. Crystals are coarse-grained (up to 70 microns reported) with perfect cleavage (Eastaugh describes as 'bladed crystals', and 'splinters'), and often a distinct 'cross-hatching' pattern is visible on larger particles. Some particles are fibrous, acicular and/or elongated, and earthy aggregates are also reported. In XPL, particles exhibit high birefringence with pink and green interference colors. Extinction is straight and acicular particles are length-fast. Synthetic orpiment can be differentiated from the naturally occurring mineral form due to its smaller particle size (reported as fine to medium). The synthetic form is also reported to be contaminated with arsenic (III) oxide. | |
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
| + | * [http://webexhibits.org/pigments/indiv/technical/orpiment.html Pigments through the ages] | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Arsenic_trisulfide Arsenic_trisulfide] (accessed Aug. 30, 2005 and March 2025) | |
| − | * Wikipedia | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 69 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 69 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 846 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 846 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | |||
| − | |||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:35, 12 March 2025
Description
A bright yellow, crystalline powder that occurs naturally as the mineral Orpiment. Orpiment is often found with lead and silver ores along with Realgar (arsenic disulfide). It was once widely used as a pigment because of its bright rich color. However, it is extremely toxic which has limited its use and availability. Arsenic trisulfide was also used to dehair and tan leathers, to kill rodents, and to print calico textiles. It is still used in fireworks and in the manufacture of infrared transmitting glass.
Synonyms and Related Terms
arsenic (III) sulfide; Pigment Yellow 39; CI 77085, 77086; orpiment (mineral); arsenic sulphide (Br.); Operment (Deut.); Rauschgelb (Deut.); Konigsgelb (Deut.); jaune royal (Fr.); trisulfure d'arsenic (Fr.); oropimente (Esp.); orpimento (It.); trisolfuro d'arsenico (It.); kio (Jap.); sekio (Jap.); ouropigmento (Port.); yellow arsenic sulfide; king's yellow; arsenic yellow; jaune royal; king's gold; arsenous sulfide; arsenic tersulfide; arsenious sulfide; auripigment
Risks
- Highly toxic by ingestion and inhalation.
- Skin contact causes irritation.
- Carcinogen and mutagen.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Physical and Chemical Properties
- Composition = As2S3 (mol. wt. = 246.04)
- CAS = 1303-33-9
- Melting Point = 310 C
- Mohs Hardness = 1.5 - 2.0
- Density = 3.43 g/ml
- Refractive Index = 2.40; 2.81; 3.02
- Boiling Point = 707 C
- Soluble in nitric acid, alkali sulfide and ammonia solutions. Insoluble in water and hydrochloric acid.
- Unstable when mixed with alkaline pigments such as in buon fresco or in combinations with lime white
- Oxidizes to form translucent or white oxides of arsenic.
- Both orpiment and realgar lose color on exposure to light
- Small, often elongated particles (4-20 microns);
- Strong yellow particles with high birefringence and straight extinction
- Luster = pearly to resinous.
- Streak = lemon-yellow.
In PPL, orpiment has a strong yellow color and exhibits very high relief. Crystals are coarse-grained (up to 70 microns reported) with perfect cleavage (Eastaugh describes as 'bladed crystals', and 'splinters'), and often a distinct 'cross-hatching' pattern is visible on larger particles. Some particles are fibrous, acicular and/or elongated, and earthy aggregates are also reported. In XPL, particles exhibit high birefringence with pink and green interference colors. Extinction is straight and acicular particles are length-fast. Synthetic orpiment can be differentiated from the naturally occurring mineral form due to its smaller particle size (reported as fine to medium). The synthetic form is also reported to be contaminated with arsenic (III) oxide.
Resources and Citations
- Pigments through the ages
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Wikipedia: Arsenic_trisulfide (accessed Aug. 30, 2005 and March 2025)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 69
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 846
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985