Difference between revisions of "Potassium ferricyanide"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Ruby red crystalline powder that is used to make [http://cameo.mfa.org/materials/fullrecord.asp?name=blueprint | + | Ruby red crystalline powder that is used to make [http://cameo.mfa.org/materials/fullrecord.asp?name=blueprint%20paper blueprints], to stain [http://cameo.mfa.org/materials/fullrecord.asp?name=wood wood], and dye [http://cameo.mfa.org/materials/fullrecord.asp?name=wool wool]. Potassium ferricyanide is also used as an [http://cameo.mfa.org/materials/fullrecord.asp?name=etching%20solution etching liquid] in electroplating and as a [http://cameo.mfa.org/materials/fullrecord.asp?name=reducing%20agent reducing agent] for photography. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 40: | Line 40: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 632 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Website address 1 Comment: Photographic chemicals at www.jetcity.com/~mrjones/chemdesc |
| − | * | + | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:32, 24 July 2013
Description
Ruby red crystalline powder that is used to make blueprints, to stain wood, and dye wool. Potassium ferricyanide is also used as an etching liquid in electroplating and as a reducing agent for photography.
Synonyms and Related Terms
farmer's reducer; potassium hexacyanoferrate (III); Mercer's liquor; red prussiate of potash; red potassium prussiate;
Other Properties
Soluble in water. Slightly soluble in ethanol.
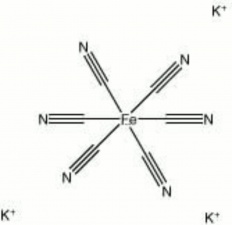
| Composition | K3Fe(CN)6 |
|---|---|
| CAS | 13746-66-2 |
| Density | 1.85 |
| Molecular Weight | mol. wt. = 329.25 |
Hazards and Safety
Decomposes with heat, acids or UV light to produce highly toxic hydrogen cyanide fumes.
Mallinckrodt Baker: MSDS
Additional Information
N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology Archetype Publications, London, 2000, p. 62.
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 632
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Website address 1 Comment: Photographic chemicals at www.jetcity.com/~mrjones/chemdesc
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000