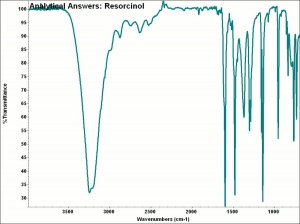
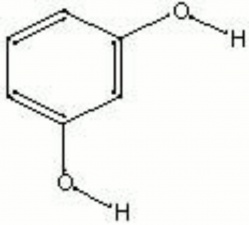
Difference between revisions of "Resorcinol"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White crystalline phenol used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=developer developer] and [http://cameo.mfa.org/materials/fullrecord.asp?name=stabilizer stabilizer] for [http://cameo.mfa.org/materials/fullrecord.asp?name=direct | + | White crystalline phenol used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=developer developer] and [http://cameo.mfa.org/materials/fullrecord.asp?name=stabilizer stabilizer] for [http://cameo.mfa.org/materials/fullrecord.asp?name=direct%20dye direct dyes]. Resorcinol also reacts with [http://cameo.mfa.org/materials/fullrecord.asp?name=formaldehyde formaldehyde], [http://cameo.mfa.org/materials/fullrecord.asp?name=acetanilide acetanilide], [http://cameo.mfa.org/materials/fullrecord.asp?name=rubber%2C%20natural rubber], and other compounds to form polymers and azo dyes. [http://cameo.mfa.org/materials/fullrecord.asp?name=resorcinol%20formaldehyde%20resin Resorcinol formaldehyde resin] cures at room temperature to form a very strong bond. It is commonly used as an [http://cameo.mfa.org/materials/fullrecord.asp?name=adhesive adhesive] for wood [http://cameo.mfa.org/materials/fullrecord.asp?name=veneer veneers] and rubber-textile [http://cameo.mfa.org/materials/fullrecord.asp?name=laminate laminates]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 44: | Line 44: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 667 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 07:33, 24 July 2013
Description
White crystalline phenol used as a developer and stabilizer for direct dyes. Resorcinol also reacts with formaldehyde, acetanilide, rubber, and other compounds to form polymers and azo dyes. Resorcinol formaldehyde resin cures at room temperature to form a very strong bond. It is commonly used as an adhesive for wood veneers and rubber-textile laminates.
Synonyms and Related Terms
resorcine; resorcin; m-dihydroxybenzene; 3-hydroxyphenol; 1,3-benzenediol
Other Properties
Soluble in water, ethanol, ether, glycerol, benzene, amyl alcohol. Slightly soluble in chloroform.
| Composition | C6H4(OH)2 |
|---|---|
| CAS | 108-46-3 |
| Melting Point | 110.7 |
| Density | 1.2717 |
| Molecular Weight | mol. wt.=110.11 |
| Boiling Point | 281 |
Hazards and Safety
Toxic by ingestion. Contact and inhalation causes severe irritation to skin, eyes and lungs.
Combustible. Flash point = 127 C (261 F)
Mallinckrodt Baker: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 667
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8323
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000