Difference between revisions of "Cellulose nitrate"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was discovered by Henri Braconnot in 1832 (xyloidine) and first commercially produced in 1838 by | + | Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was discovered by Henri Braconnot in 1832 (xyloidine) and first commercially produced in 1838 by Théophile-Jules Pelouze as an explosive (nitramidine). As production techniques improved, nitrocellulose was developed as a plastic, especially as a substitute for [http://cameo.mfa.org/materials/fullrecord.asp?name=ivory ivory]. It was marketed in the U.S. as [http://cameo.mfa.org/materials/fullrecord.asp?name=Celluloid Celluloid], a proprietary mixture of cellulose nitrate with [http://cameo.mfa.org/materials/fullrecord.asp?name=camphor camphor] as a plasticizer. Celluloid was molded into numerous shapes such as piano keys, billiard balls, ping pong balls, dolls, buttons and boxes. It was used to make inexpensive objects and decorations that imitated the appearance of ivory, [http://cameo.mfa.org/materials/fullrecord.asp?name=amber amber], [http://cameo.mfa.org/materials/fullrecord.asp?name=carnelian carnelian], [http://cameo.mfa.org/materials/fullrecord.asp?name=coral coral], [http://cameo.mfa.org/materials/fullrecord.asp?name=seashell seashell] and [http://cameo.mfa.org/materials/fullrecord.asp?name=tortoiseshell tortoiseshell]. Cellulose nitrate was also used for photographic film (from the 1880s to 1920s) and animated drawing cels. Because of its instability, its use for cinematography was limited in 1912 and banned in 1951. In the early 20th century, cellulose nitrate was often used for clear lacquers, fabric dopes, adhesives, and high-gloss paints. During the 1940s and 50s, cellulose nitrate was commercially sold in mixed with natural resins ([http://cameo.mfa.org/materials/fullrecord.asp?name=dammar dammar], [http://cameo.mfa.org/materials/fullrecord.asp?name=shellac shellac], [http://cameo.mfa.org/materials/fullrecord.asp?name=copal copal], etc) as a waterproof varnish. Cellulose nitrate is inherently unstable and slowly decomposes at room temperature. Ultraviolet light, heat, and/or high humidities hasten its decomposition. Cellulose nitrate is still sold in adhesives, coatings, and explosives. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | nitrocellulose; Zellulosenitrat (Deut.); nitrocellulose (Fr.); fulmicoton (Fr.); nitrocellulosa (It.); schietkatoen (Ned.); cellulosenitraat (Ned.); nitroceluloza (Pol.); nitrato de celulosa (Esp.); nitrocelulosa (Esp.); nitrato di cellulosa, celluloide (It.); nitrocelulose (Port.); trinitrocelulose (Port.); | + | nitrocellulose; Zellulosenitrat (Deut.); nitrocellulose (Fr.); fulmicoton (Fr.); nitrocellulosa (It.); schietkatoen (Ned.); cellulosenitraat (Ned.); nitroceluloza (Pol.); nitrato de celulosa (Esp.); nitrocelulosa (Esp.); nitrato di cellulosa, celluloide (It.); nitrocelulose (Port.); trinitrocelulose (Port.); algodão-pólvora (Port.); bomullskrut (Sven.); nitrocellulosakrut (Sven.); nitrerad cellulosa (Sven.); nitro-cellulose; chardonnet; French ivory; pyroxylin; airplane wing dope, guncotton; gun-cotton; collodion; celloidin; celluidine; nitrocotton; photoxylin |
| − | Examples: Celluloid; Xyloidine; Nitramidine; Parkesine; Zapon-lack [Dulux]; HMG [H.Marcel Guest]; Durofix [Rawplug]; | + | Examples: Celluloid; Xyloidine; Nitramidine; Parkesine; Zapon-lack [Dulux]; HMG [H.Marcel Guest]; Durofix [Rawplug]; Duco® cement [DuPont]; UHU Hart [Linger & Fischer, Germany]; |
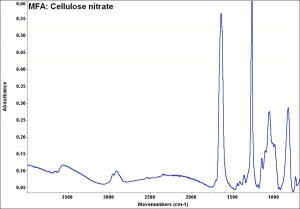
[[[SliderGallery rightalign|MFA- Cellulose nitrate.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Cellulose nitrate.jpg~FTIR]]] | ||
| Line 37: | Line 37: | ||
== Additional Information == | == Additional Information == | ||
| − | J.Reilly, "Celluloid Objects: Their Chemistry and Preservation" JAIC, 145-162, 1991. [http://aic.stanford.edu/jaic/articles/jaic30-02-003.html Link] | + | ° J.Reilly, "Celluloid Objects: Their Chemistry and Preservation" JAIC, 145-162, 1991. [http://aic.stanford.edu/jaic/articles/jaic30-02-003.html Link] ° Museum Handbook, Part 1. Museums Collections. Web Edition. Appendix M. Management of Cellulose Nitrate and Cellulose Acetate Films, NPS, 2001. [http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf] |
== Comparisons == | == Comparisons == | ||
| Line 49: | Line 49: | ||
== Authority == | == Authority == | ||
| − | * | + | * M.Kaufman, ''The First Century of Plastics'', The Plastics and Rubber Institute, London, 1963 |
| − | * | + | * Website address 1 Comment: History of Plastics: www.nswpmith.com.au/historyofplastics.html |
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cellulose_nitrate (Accessed Jan. 15, 2006) - first discovered by Henri Braconnot, a French chemist, in 1832 | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cellulose_nitrate (Accessed Jan. 15, 2006) - first discovered by Henri Braconnot, a French chemist, in 1832 | ||
| − | * | + | * B. Gascoigne, ''How to Identify Prints'', Thames & Hudson, London, 2004 |
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 171 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * C.V.Horie, ''Materials for Conservation'', Butterworth-Heineman, London, 1997 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 |
| − | * | + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 |
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"; "Ivory" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"; "Ivory" | ||
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:56, 24 July 2013
Description
Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was discovered by Henri Braconnot in 1832 (xyloidine) and first commercially produced in 1838 by Théophile-Jules Pelouze as an explosive (nitramidine). As production techniques improved, nitrocellulose was developed as a plastic, especially as a substitute for ivory. It was marketed in the U.S. as Celluloid, a proprietary mixture of cellulose nitrate with camphor as a plasticizer. Celluloid was molded into numerous shapes such as piano keys, billiard balls, ping pong balls, dolls, buttons and boxes. It was used to make inexpensive objects and decorations that imitated the appearance of ivory, amber, carnelian, coral, seashell and tortoiseshell. Cellulose nitrate was also used for photographic film (from the 1880s to 1920s) and animated drawing cels. Because of its instability, its use for cinematography was limited in 1912 and banned in 1951. In the early 20th century, cellulose nitrate was often used for clear lacquers, fabric dopes, adhesives, and high-gloss paints. During the 1940s and 50s, cellulose nitrate was commercially sold in mixed with natural resins (dammar, shellac, copal, etc) as a waterproof varnish. Cellulose nitrate is inherently unstable and slowly decomposes at room temperature. Ultraviolet light, heat, and/or high humidities hasten its decomposition. Cellulose nitrate is still sold in adhesives, coatings, and explosives.
Synonyms and Related Terms
nitrocellulose; Zellulosenitrat (Deut.); nitrocellulose (Fr.); fulmicoton (Fr.); nitrocellulosa (It.); schietkatoen (Ned.); cellulosenitraat (Ned.); nitroceluloza (Pol.); nitrato de celulosa (Esp.); nitrocelulosa (Esp.); nitrato di cellulosa, celluloide (It.); nitrocelulose (Port.); trinitrocelulose (Port.); algodão-pólvora (Port.); bomullskrut (Sven.); nitrocellulosakrut (Sven.); nitrerad cellulosa (Sven.); nitro-cellulose; chardonnet; French ivory; pyroxylin; airplane wing dope, guncotton; gun-cotton; collodion; celloidin; celluidine; nitrocotton; photoxylin
Examples: Celluloid; Xyloidine; Nitramidine; Parkesine; Zapon-lack [Dulux]; HMG [H.Marcel Guest]; Durofix [Rawplug]; Duco® cement [DuPont]; UHU Hart [Linger & Fischer, Germany];
Other Properties
Birefringent. Softening point 155-200C. No electical charge when rubbed with silk.
Soluble in ketones, esters, and ether alcohol mixtures. Insoluble in water, ethanol, and hydrocarbons. Burns with a bright, violent flame; smells of nitrogen oxides.
One drop of diphenylamine solution (6% in conc. sulfuric acid) gives positive deep blue color for cellulose nitrate
| Density | 1.34-1.40 |
|---|---|
| Refractive Index | 1.49-1.51 |
Hazards and Safety
Highly flammable. Explosion risk. Flash point = 13C (55F)
Ultraviolet light, high temperatures and moisture accelerate degradation. May adversely react with metals (lead, silver, tin, iron, copper and zinc).
Additional Information
° J.Reilly, "Celluloid Objects: Their Chemistry and Preservation" JAIC, 145-162, 1991. Link ° Museum Handbook, Part 1. Museums Collections. Web Edition. Appendix M. Management of Cellulose Nitrate and Cellulose Acetate Films, NPS, 2001. http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Authority
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- Website address 1 Comment: History of Plastics: www.nswpmith.com.au/historyofplastics.html
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Cellulose_nitrate (Accessed Jan. 15, 2006) - first discovered by Henri Braconnot, a French chemist, in 1832
- B. Gascoigne, How to Identify Prints, Thames & Hudson, London, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 171
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"; "Ivory"
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988