Difference between revisions of "Pyridine"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A yellow liquid with a noxious odor. Pyridine was first prepared from coal-tar in 1846 by Anderson. Pyridine is used as a [ | + | A yellow liquid with a noxious odor. Pyridine was first prepared from coal-tar in 1846 by Anderson. Pyridine is used as a [[solvent|solvent]] and it is one of the few solvents that can dissolve dried [[linseed%20oil|linseed oil]] in paints and varnishes. It is used as a denaturant for [[ethyl%20alcohol|ethanol]] to make it unfit for drinking. Pyridine has also been used as a chemical raw material for manufacturing many compounds and as a water repellent, bactericide, and herbicide. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 11:39, 10 May 2016
Description
A yellow liquid with a noxious odor. Pyridine was first prepared from coal-tar in 1846 by Anderson. Pyridine is used as a Solvent and it is one of the few solvents that can dissolve dried Linseed oil in paints and varnishes. It is used as a denaturant for ethanol to make it unfit for drinking. Pyridine has also been used as a chemical raw material for manufacturing many compounds and as a water repellent, bactericide, and herbicide.
Synonyms and Related Terms
azabenzene; azine
Other Properties
Soluble in water, ethanol, ether, benzene, ligroin and fatty oils.
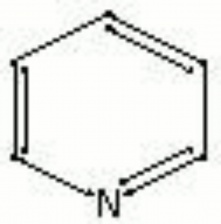
| Composition | N(CH)4CH |
|---|---|
| CAS | 110-86-1 |
| Melting Point | -42.0 |
| Density | 0.987 |
| Molecular Weight | mol. wt.=79.11 |
| Refractive Index | 1.5092 |
| Boiling Point | 115.5 |
Hazards and Safety
Flammable. Flash point = 68F. Dangerous fire risk. Combustion produces highly toxic cyanide gases.
Toxic by ingestion and inhalation. May be absorbed through the skin causing irritation.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.817
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- George Savage, Art and Antique Restorer's Handbook, Rockliff Publishing Corp, London, 1954
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 7869; ref. index=1.5092
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.507