Difference between revisions of "Charcoal"
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:28.636-SC71899.jpg|thumb|]] | + | [[File:28.636-SC71899.jpg|thumb|Sketch by Sargent<br>MFA# 28.636]] |
== Description == | == Description == | ||
| Line 13: | Line 13: | ||
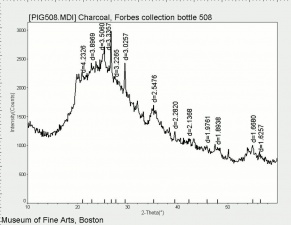
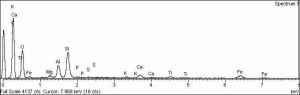
[[[SliderGallery rightalign|PIG508.jpg~XRD|f508sem.jpg~SEM|f508edsbw.jpg~EDS]]] | [[[SliderGallery rightalign|PIG508.jpg~XRD|f508sem.jpg~SEM|f508edsbw.jpg~EDS]]] | ||
| + | == Risks == | ||
| − | == | + | Fire risk. May ignite spontaneously in air. |
| + | == Physical and Chemical Properties == | ||
Microscopically, tiny wood splinters may be visible. | Microscopically, tiny wood splinters may be visible. | ||
| Line 23: | Line 25: | ||
| oak=0.57; pine=0.28-0.44 | | oak=0.57; pine=0.28-0.44 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
| Line 37: | Line 35: | ||
</gallery> | </gallery> | ||
| − | + | == Resources and Citations == | |
| − | == | ||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 182 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 182 | ||
| Line 46: | Line 43: | ||
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Charcoal (Accessed Sept. 2 2005) |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density for oak=0.57; pine=0.28-0.44 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density for oak=0.57; pine=0.28-0.44 | ||
Latest revision as of 12:13, 15 August 2020
Description
A black, porous carbonaceous material. Charcoal is the Carbon containing residue from burned wood (e.g., Willow, Maple, Beech, Linden or plum) or other organic containing materials such as bone, plants or animals. Charcoal contains 80 to 98% carbon with some ash and moisture. Charcoal has been used since ancient times as a drawing material and pigment (see Charcoal black). Charcoal is also sold commercially as a fuel, abrasive, sorbent, filter media, and decolorizer.
See also Activated carbon, and Charcoal crayon.
Synonyms and Related Terms
trækul (Dan.); negro carbón (Esp.); carbón vegetal (Esp.); Holzkohle (Deut.); charbon de bois (Fr.); karboyno (Gr.); carbonella (It.); carbone (It.); carbo ligni (Lat.); houtskool zwart (Ned.); carvão vegetal (Port.)
Risks
Fire risk. May ignite spontaneously in air.
Physical and Chemical Properties
Microscopically, tiny wood splinters may be visible.
| Density | oak=0.57; pine=0.28-0.44 |
|---|
Additional Images
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 182
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Wikipedia: http://en.wikipedia.org/wiki/Charcoal (Accessed Sept. 2 2005)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density for oak=0.57; pine=0.28-0.44
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998