Difference between revisions of "Tannin"
Jump to navigation
Jump to search
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Any of several compounds used for dyeing cloth and tanning leather. Tannins are usually a catechol, pyrogallol, phenolic, or aldehyde type compounds. Natural tannins are found in many plants and are usually concentrated in bark and damaged tissues such as galls and wounds. Tannins reacts with proteins ([[animal%20skin| | + | Any of several compounds used for dyeing cloth and tanning leather. Tannins are usually a catechol, pyrogallol, phenolic, or aldehyde type compounds. Natural tannins are found in many plants and are usually concentrated in bark and damaged tissues such as galls and wounds. Tannins reacts with proteins ([[animal%20skin|Skin]], [[albumin|albumin]], [[gelatin|gelatin]], etc.) to form insoluble products that are resistant to degradation. Skin treated with tannins is called [[leather|leather]]. Tannins have been used for several thousand years, especially in China. Examples of natural tannins are [[tannic%20acid|tannic acid]], [[myrobalan%20extract|myrobalans]], and [[quebracho|quebracho]]. Examples of synthetic tannins are [[formaldehyde|formaldehyde]], [[glutaraldehyde|glutaraldehyde]], and [[hydroxyacetic%20acid|glycolic acid]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
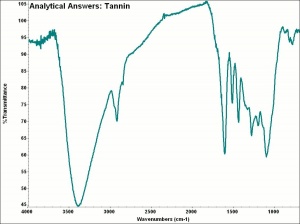
[[[SliderGallery rightalign|aaiTANNIN.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiTANNIN.jpg~FTIR]]] | ||
| − | == | + | == Physical and Chemical Properties == |
| − | Soluble in water or ethanol | + | * Soluble in water or ethanol |
| + | * Insoluble in ether, chloroform, carbon disulfide, benzene | ||
| + | * Precipitates gelatin. | ||
| − | == | + | == Resources and Citations == |
| − | Ann Hagerman, Tannin Handbook, at [http://www.users.muohio.edu/hagermae/tannin.pdf Website] (provides extensive information on tannin chemistry and analysis) | + | * Ann Hagerman, Tannin Handbook, at [http://www.users.muohio.edu/hagermae/tannin.pdf Website] (provides extensive information on tannin chemistry and analysis) |
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 796 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 796 | ||
Latest revision as of 16:33, 30 October 2020
Description
Any of several compounds used for dyeing cloth and tanning leather. Tannins are usually a catechol, pyrogallol, phenolic, or aldehyde type compounds. Natural tannins are found in many plants and are usually concentrated in bark and damaged tissues such as galls and wounds. Tannins reacts with proteins (Skin, Albumin, Gelatin, etc.) to form insoluble products that are resistant to degradation. Skin treated with tannins is called Leather. Tannins have been used for several thousand years, especially in China. Examples of natural tannins are Tannic acid, myrobalans, and Quebracho. Examples of synthetic tannins are Formaldehyde, Glutaraldehyde, and glycolic acid.
Synonyms and Related Terms
Tannine (Deut., Ned.); tanin (Fr.); tannin (Sven.)
Physical and Chemical Properties
- Soluble in water or ethanol
- Insoluble in ether, chloroform, carbon disulfide, benzene
- Precipitates gelatin.
Resources and Citations
- Ann Hagerman, Tannin Handbook, at Website (provides extensive information on tannin chemistry and analysis)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 796
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "tannin" [Accessed 28 Sept. 2005].
- Wikipedia: http://en.wikipedia.org/wiki/Tannin (Accessed Sept. 28, 2005)