Difference between revisions of "Aniline sulfate"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A toxic, white powder that is prepared by heating [ | + | A toxic, white powder that is prepared by heating [[aniline|aniline]] with [[sulfuric%20acid|sulfuric acid]]. A 1% solution of aniline sulfate can be used as a reagent for the detection of mechanical pulp paper. Lignin containing fibers, such as groundwood or jute, give a positive yellow color reaction. [[Cotton|Cotton]], [[linen|linen]], and [[hemp|hemp]] turn the solution brown. A pink color is a positive reaction for the presence of [[esparto%20grass|esparto grass]] (Roberts and Etherington 1982). |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
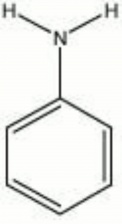
[[[SliderGallery rightalign|aniline sulfate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|aniline sulfate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Toxic by inhalation, ingestion and skin contact. Very toxic to aquatic organisms. Light sensitive. Hygroscopic. Combustible. | ||
| + | |||
| + | Fisher Scientific: [https://fscimage.fishersci.com/msds/27886.htm MSDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water. | Soluble in water. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.38 | + | | 1.38 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 34: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 11687 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 11687 | ||
Latest revision as of 10:03, 24 April 2022
Description
A toxic, white powder that is prepared by heating Aniline with Sulfuric acid. A 1% solution of aniline sulfate can be used as a reagent for the detection of mechanical pulp paper. Lignin containing fibers, such as groundwood or jute, give a positive yellow color reaction. Cotton, Linen, and Hemp turn the solution brown. A pink color is a positive reaction for the presence of Esparto grass (Roberts and Etherington 1982).
Synonyms and Related Terms
aniline sulphate (Br.)
Risks
Toxic by inhalation, ingestion and skin contact. Very toxic to aquatic organisms. Light sensitive. Hygroscopic. Combustible.
Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in water.
| Composition | (C6H5NH2)2-H2SO4 |
|---|---|
| CAS | 542-16-5 |
| Density | 1.38 g/ml |
| Molecular Weight | mol. wt. = 284.33 |
Resources and Citations
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 11687
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Aldrich Chemical Catalog