Difference between revisions of "Ammonium phosphate dibasic"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 32: | Line 32: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | Harmful if swallowed. Contact causes irritation. | + | * Harmful if swallowed. |
| − | + | * Contact causes irritation. | |
| − | + | * Fisher Scientific: [https://www.fishersci.com/store/msds?partNumber=AC447140010&productDescription=AMMONIUM+PHOSPHATE%2C+DIBA+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | |
== Additional Information == | == Additional Information == | ||
Revision as of 13:26, 26 April 2022
Description
Ordorless, colorless crystals that is used to fireproof textiles, paper, wood, and baskets. Ammonium phosphate dibasic is also used as a flux for soldering Tin, Copper, Brass, and Zinc.
Synonyms and Related Terms
secondary ammonium phosphate; diammonium hydrogen phosphate
Other Properties
Soluble in water forming solution with pH about 8.
Insoluble in alcohol, acetone.
Used for the detection of magnesium (Anrnold 1994).
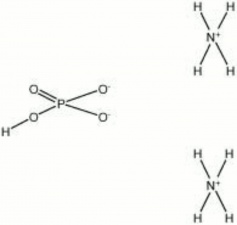
| Composition | (NH4)2HPO4 |
|---|---|
| CAS | 7783-28-0 |
| Density | 1.62 |
| Molecular Weight | mol. wt. = 132.06 |
Risks
- Harmful if swallowed.
- Contact causes irritation.
- Fisher Scientific: SDS
Additional Information
° Arnold, Studies in Conservation (29) 1994
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 576
- External source or communication Comment: detection of magnesium - Arnold, Studies in Conservation (29) 1994
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Ammonium_phosphate (Accessed Mar. 20, 2006)