Difference between revisions of "Antimony pentoxide"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 9: | Line 9: | ||
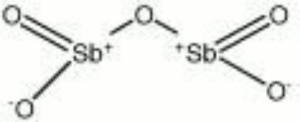
[[[SliderGallery rightalign|antimony pentoxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|antimony pentoxide.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Decomposes on heating. | ||
| + | * Strong oxidizer. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/98839.htm MSDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in strong bases and concentrated hydrochloric acid. Slightly soluble in water. | Soluble in strong bases and concentrated hydrochloric acid. Slightly soluble in water. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 450 | + | | 450 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.78 | + | | 3.78 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 13:27, 27 April 2022
Description
Yellowish powder with cubic crystals. Antimony pentoxide is used as a Flame retardant in clothing.
Synonyms and Related Terms
antimony (V) oxide; diaphoretic antimony; white oxide of antimony; antimony anhydride; antimonic acid; stibic anhydride
Risks
- Decomposes on heating.
- Strong oxidizer.
- Fisher Scientific: MSDS
Physical and Chemical Properties
Soluble in strong bases and concentrated hydrochloric acid. Slightly soluble in water.
| Composition | Sb2O5 |
|---|---|
| CAS | 1314-60-9 |
| Melting Point | 450 C |
| Density | 3.78 g/ml |
| Molecular Weight | mol. wt. = 323.52 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 739
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985